-
Glutamate is an amino acid and one of the basic components of protein. Upon protein intake, a large amount of glutamic acid is produced in the body after digestion and absorption. Its sodium salt is widely used to improve the umami taste of food, thereby increasing appetite and food intake. A Japanese professor stumbled upon the fact that Japanese wood fish and kelp dashi both have a special taste that is different from sweet, salty, sour, and bitter[1]. Using water extraction and crystallization, monosodium glutamate (MSG) was isolated from kelp, presenting a new type of seasoning that has expanded in the food industry. In China, the production of MSG started in 1921 by Wu, further to emerge into a stage of rapid development since the 1980s, as a major producer of MSG in the world. Food condiments such as soy sauces and chicken essences also contain certain levels of MSG via protein fermentation or chemical addition[2].
As an umami taste modulator, it mainly binds to taste receptor heterodimers (TAS1R1-TAS1R3), activates a series of metabolic actions, increasing the release of intracellular calcium, thus activating the taste afferents by depolarization followed by generation of action potentials acting on purinergic receptors[3].
At present, the usage regulations on MSG in different countries around the world are somewhat different. In some countries, especially in Asia, people like to add MSG to enhance the taste upon cooking. However, MSG is prohibited from being used in some European and American countries, such as USA and Mexico. The use of MSG remains a controversial topic owing to its side effects. MSG side effects were first reported in 1968 which manifested as palpitations, numbness, and weakness in the back of the neck and arms, later known as MSG syndrome[4]. In addition, numerous studies showed that MSG intake may increase the potential risk of nerve[5] and gastrointestinal diseases[6]. Meanwhile, it was forbidden to add MSG to baby food dating back to the late 1970s. Pregnant women are also advised not to consume MSG-containing foods during pregnancy or breastfeeding[7]. This article reviews the absorption and metabolism of MSG inside the body, and its safety to the human body, and provides updated information on the application of MSG as a food additive in human health.
-
MSG has been identified and recognized for over 100 years. In 1861, a German professor extracted glutamic acid from wheat gluten for the first time. Subsequently, a Japanese professor identified the glutamic acid from the juice of kelp in 1908. Japan began to produce and sell the first commercial MSG under the trademark 'Ajinomoto' in 1909. Since then, MSG has been used as a food additive to enhance the flavor of food and has been widely used all over the world, especially in Asian countries. So far, there have been a variety of methods proven effective to produce MSG including separation and extraction, chemical synthesis, and fermentation. The advantages or disadvantages of various methods of producing MSG are summarized in Table 1.
Table 1. The advantages or disadvantages of various methods for producing MSG.
Separation
and extractionChemical synthesis Fermentation Materials Wheat
glutenAcrylonitrile Selected
microorganism,
carbohydrates
and ammoniaYield ↑ × √ √ Time ↓ × √ √ Purity ↑ × √ √ Cost ↓ × √ √ Environmentally friendly ↑ × × √ MSG was also found in many natural foods, such as tomatoes, fermented soy products, asparagus, mushrooms, walnuts, and milk to impart a special flavor (called umami) that exists in addition to the four classic flavors (sour, sweet, bitter, and salty)[2]. Currently, it is added to various processed foods, such as snacks, fast food, soups and various canned foods. As an umami taste regulator, it will bind to the TAS1R1-TAS1R3 to activate the taste G protein (α-gustducin), leading to the activation of phospholipase Cβ2 and inositol triphosphate, thereby increasing intracellular calcium release. Calcium activates the transient receptor potential cation channel M5, which then depolarizes and releases adenosine triphosphate to generate an action potential. Adenosine triphosphate acts on purinergic receptors, and triggers the taste afferents. Finally, the taste center of the brain is stimulated, in a way that the umami taste brought by MSG is recognized[8].
-
MSG is mainly absorbed by active transport inside the small intestine. The glutamate in the intestinal lumen is transported across the apical membrane to the intestinal epithelial cells by glutamate transporter. This transport mainly relies on the high-affinity X−AG system, which is mainly composed of solute carrier family 1 member 1 (SLC1A1), SLC1A2, SLC1A3, SLC1A6 and SLC1A7[9]. The absorption and transport of glutamate in the intestinal tract mainly depends on SLC1A1[10]. Consequently, the regulation of its expression level and transport activity directly affects the absorption, and function of glutamate.
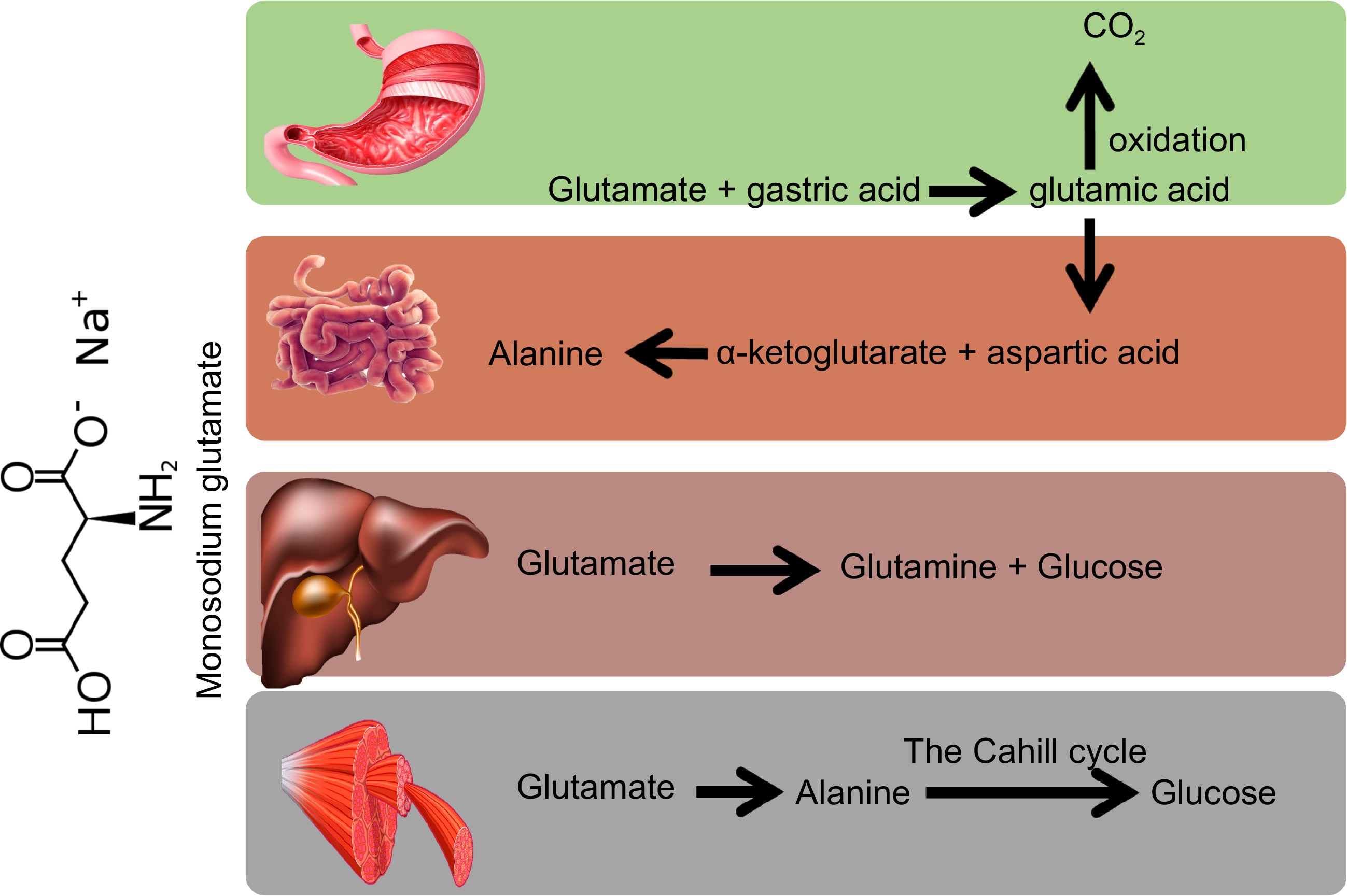
After MSG is ingested inside the human body, MSG reacts with gastric acid to produce glutamic acid and sodium chloride, which are quickly digested and absorbed, and participate in various metabolic reactions. Studies have proved that 13C-labeled glutamate directly fed into piglets stomachs, was oxidized to CO2 as the main metabolic product[11]. Specifically, glutamic acid could be metabolized in the stomach. In addition, the intestine is also considered to be one of the main sites of glutamate absorption[12]. The metabolic pathway of glutamic acid in the small intestine is described as glutamic acid that yields α-ketoglutarate and aspartic acid through transamination between epithelial cells and oxaloacetate. In the presence of pyruvate, glutamic acid can produce alanine and α-ketoglutarate through transamination. The α-ketoglutarate produced by transamination could enter the mitochondria, to produce a reducing coenzyme for the synthesis of mitochondrial ATP through the tricarboxylic cycle. The aspartic acid produced by transamination of glutamate could likewise enter the mitochondria and can be oxidized through the tricarboxylic acid cycle[13]. The above observations indicated that glutamate is not only an important energy substrate in the intestine, but also a prerequisite for some other amino acids and active substances, and it can play an important role in the activities of the digestive tract.
Although glutamate is mainly metabolized in the stomach and small intestine, it was also metabolized in tissues such as liver and muscle. Inside the liver, glutamate is converted to glucose, glutamine (Gln) or other amino acids[14]. Whereas in the muscle tissue, on the one hand, glutamate generated alanine through transamination reaction, then, it produces glucose through the Cahill cycle. On the other hand, glutamate was converted to Gln by the enzyme Gln synthetase[15] (Fig. 1).
-
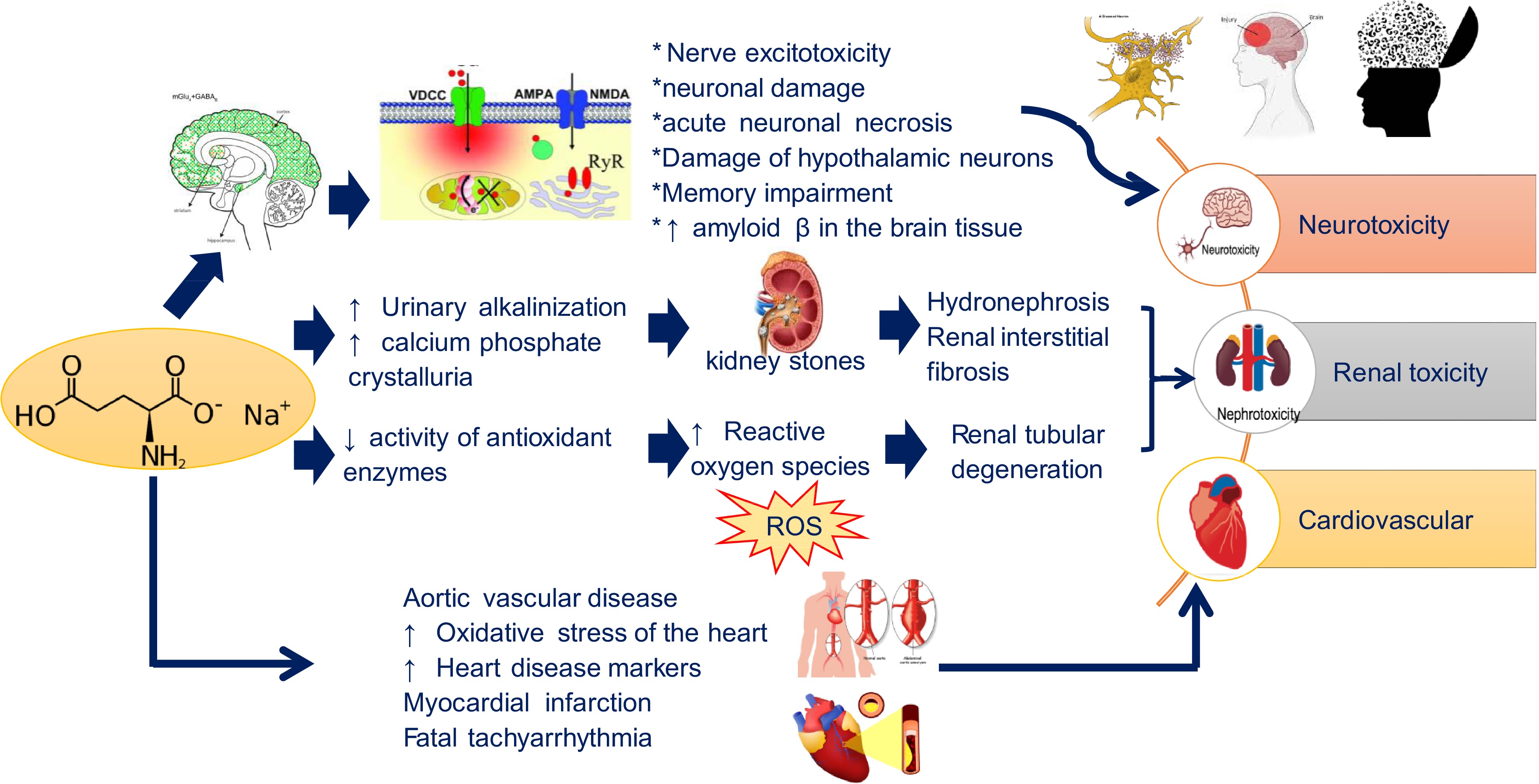
In recent years, the safety of MSG has attracted widespread attention. As the precursor of Gln, proline and arginine, glutamic acid is a coded amino acid in the process of protein synthesis and likewise a non-essential amino acid in mammals[16]. Although the use of MSG has shown no clear harmful effects on the human body since its discovery, MSG has caused much controversy in recent years. In 1968, the New England Journal of Medicine first reported the adverse effects of general weakness, heart palpitations, and transient numbness of limbs after eating MSG and called it the 'Chinese Restaurant Syndrome'[4]. In addition, the results of animal experiments also showed that MSG could induce obesity, non-alcohol fatty liver diseases (NAFLD), neurotoxicity, etc. Therefore, the safety of MSG is still a controversial topic warranting critical analysis of its health effects targeting different organs as highlighted in this review (Fig 2).
Metabolic syndrome
-
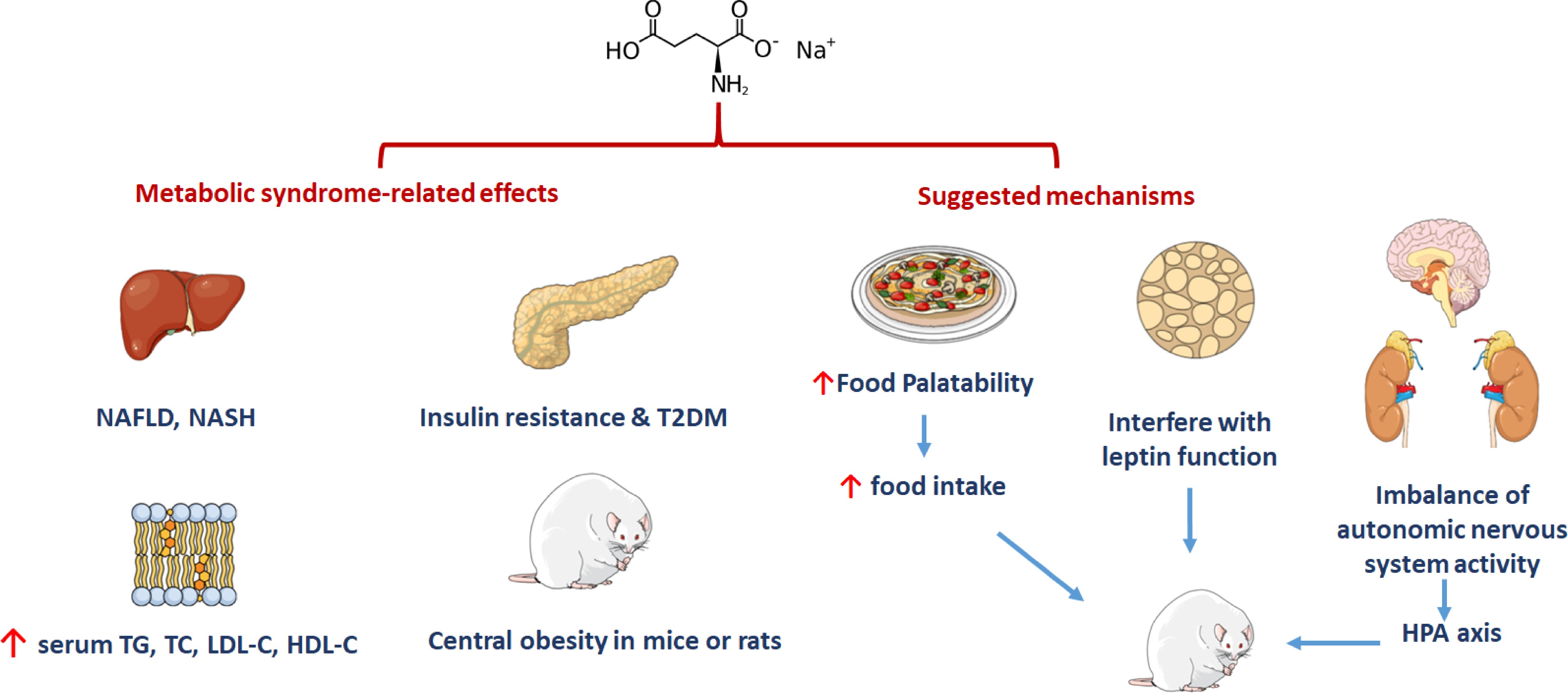
Metabolic syndrome refers to the pathological condition, in which the body’s protein, fat, carbohydrates and other substances are metabolically disordered. It is a complex of metabolic disorders (such as obesity, insulin resistance, hypertension, atherogenic dyslipidemia, NAFLD, etc.) that is considered to be one of the risk factors for cardiovascular disease. The incidence of metabolic syndrome is increasing year on year worldwide. Studies have shown that metabolic syndrome is not only related to excessive calorie intake, but also some specific diets can often induce metabolic syndrome. Some in vivo studies reported that MSG could lead to metabolic syndrome (Fig 3). Numerous animal experiments have shown that gavage or subcutaneous injection of MSG could induce central obesity in mice or rats[17], as well as NAFLD, Non-alcoholic steatohepatitis (NASH)[18], insulin resistance[19], type 2 diabetes mellitus (T2DM)[20]. The specific effects of MSG on metabolic syndrome are summarized in Table 2.
Table 2. List of the effect of MSG on metabolic syndrome.
Diseases Dosage/day Species Effects References Obesity Inject MSG (4 mg/g) Mice Induced obesity [18] Mixed with 3, 6 and 9 g of MSG Wistar rats Increased in body weight gain [88] S.c. 4 mg/g Adult Wistar rats Induced obesity [23] 2.2 ± 1.6 g/d Healthy Chinese adults Induced overweight [26] 250 g of MSG for food preparation
for 10 dFamilies from rural area of Thailand Overweight [27] NAFLD S.c. 2 mg/g ICR mice Induced obesity and diabetes with steatosis and steatohepatitis resembling human NAFLD and NASH with pre-neoplastic lesions [33] Drinking water containing
0.64 g/L MSGFemale C57BL/6J mice Induced dyslipidemia and markers of insulin resistance [19] Insulin resistant S.c. 4 g/kg Newborn Wistar rats Developed glucose intolerance [20] T2DM S.c. 4 mg/g Male DIAR mice Obesity, diabetes and macrovesicular steatohepatitis [21] In addition, it has also been found that the maternal intake of high-dose MSG during pregnancy is also related to neonatal metabolic syndrome. Some animal studies in cats revealed that when mothers consumed a diet supplemented with 1.125% MSG during pregnancy, liver degeneration of the kittens occurred, as well as an increase in serum triglyceride, total cholesterol, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol levels. Insulin resistance also occurred in the kittens[21]. Similarly, high-dose MSG intake during rodents’ pregnancy could lead to obesity of offspring, which is mainly manifested as dyslipidemia, hyperglycemia, hyperinsulinemia, and impaired leptin signaling[22]. Whether such effects could also be reflected in humans has yet to be concluded. MSG is reported to induce metabolic syndrome attributed to its use as a common flavor enhancer causing increase in the palatability of food, thereby greatly increasing food intake. On the other hand, MSG could interfere with the function of leptin, which is a hormone that responsible for shutting down the appetite via signaling to the brain when full[23]. Impairment of the hypothalamic-pituitary axis (HPA) pathway can lead to an imbalance of autonomic nervous system activity in MSG-treated rats, contributing to the development of obesity[24]. In terms of clinical research, it is found that, regardless of the total calorie intake and the amount of exercise, the intake of MSG will increase the risk of obesity in healthy Chinese subjects[25]. Insawang et al. studied the relationship between MSG intake and metabolic syndrome in rural Thailand populations revealing that every 1 g increase in MSG daily intake, accelerates the risk of metabolic syndrome by 1.14 times, whereas the risk of obesity was increased by 1.16 times[26].
Neurotoxicity
-
Neurotoxicity refers to damage to the structure or function of the nervous system caused by foreign chemicals[27]. As an excitatory amino acid with high content in human and mammalian brains, glutamate is involved in the transmission of information in the central nervous system. Glutamate receptors such as N-methyl-D-aspartate (NMDA) receptors are widely distributed in the central nervous system including the amygdala, hippocampus and hypothalamus, and are involved in the regulation of energy metabolism and autonomous functions. If an excessive amount of MSG (4 mg/g) is ingested, glutamate will enter the brain in large quantities, and the concentration will rise sharply, which could ultimately lead to nerve excitotoxicity, neuronal damage or death, with symptoms such as dizziness, panic attacks, and asthma[28].
The neurotoxicity of MSG was first reported by Olney in 1969 where the injection of MSG into newborn mice caused an acute neuronal necrosis[29]. Burde et al. also confirmed in 1971 that oral and subcutaneous injection of MSG (2 mg/g or 4 mg/g) in rats could cause acute necrotizing damage to hypothalamic neurons[30]. Meanwhile, many studies have revealed that the use of different doses of MSG produced different neurological phenotypes[31]. Continuous intragastric administration of 2 g/kg of MSG in Wistar rats could cause memory impairment and inhibit Na+-K+-ATPase in the hippocampus and cortex[32]. Newborn SD rats subcutaneously injected with MSG (4 mg/kg) on day 1, 3, 5, 6, 7 and 9, respectively showed Alzheimer-like disabilities and memory impairment, as well as hippocampal synapse-related proteins misfunction, while the phosphorylated tau protein level showed an increase[20]. Rats administered MSG (100 mg/kg/d) for two consecutive months, also have elevated levels of amyloid β in the brain tissue, concurrent with an increase in Na+, Ca2+, K+ levels. The results of hematoxylin and eosin (H&E) stain showed that severe neuronal degeneration, perivascular edema, hyperemia, and nuclear condensation occurred in the brain tissue[33]. In adult mice and rats, subcutaneous injections of MSG caused rapid degeneration of neurons. Acute necrosis of acetylcholinesterase-positive neurons in the final zone was observed in both the mother rats administered with MSG during pregnancy and their offspring, with offspring’s neurons found more sensitive to MSG[34].
Because the blood-brain barrier is immature in neonates, once a large dose of MSG is taken, it will seriously affect the learning ability, concurrent with a dysregulation of the rhythm of the hippocampus and the frontal cortex[35]. The specific changes in the central nervous system are summarized in Table 3.
Table 3. The effect of MSG on nervous system and kidneys.
Diseases Dosage/d Species Effects References Nervous system S.c 4 mg/g Male Wistar rats Increased brain extracellular Glu level and produced hyperexcitability and motor behavior alterations [30] 3,200 mg/kg/d − Induced neurodevelopmental toxicity [9] 2 mg/g or 4 mg/g Mice and rats Caused acute neuronal necrosis [31] I.p.500 mg/kg Adult male Fischer-344 rats Altered the dopamine content in adult rats [32] I. p. 2 g/kg Male Wistar rats Lead to long-term memory impairment [34] − Mice and rats Exhibited peripheral insulin resistance, cognitive deficits, and a reduction in the total hippocampal volume with decreased neuron count in the DG, CA3, and CA1 subfields. [35] Kidneys 4 mg/g Adult albino Wistar rats The cortex of the kidneys developed variable pathological changes [37] 3 g/kg Male Wistar rats Produced hyperfiltration with increased tubular reabsorption of Na, K and water [38] 2 mg/g Wistar male rats The renal handling and toxicity mainly related to oxidative stress and metabolism [39] 3 g/kg Male Wistar rats Occurred the renal pathologic changes, intrarenal oxidative stress and reduction of nitric oxide excretion [40] Renal toxicity
-
As one of the important organs of the human body, the main function of the kidneys is to produce urine, eliminate metabolites, waste and poisons in the body, as well as retain water and other useful substances through reabsorption to regulate water, electrolyte and pH balance. Consequently, kidneys are one of the organs most affected by toxic substances such as MSG.
Preclinical studies have shown that excessive intake of MSG (2 mg/g−4 g/kg) in rats can lead to urinary alkalization and calcium phosphate crystalluria, which will lead to the formation of kidney stones, followed by hydronephrosis and renal interstitial fibrosis[36]. Meanwhile, sodium reabsorption, glomerular filtration rate and renal cortex plasma flow increased after giving free food (3 g/kg/d) and water (1% MSG) to 5-week-old Wistar rats for 16 weeks. H&E stain examination further showed that NMDA receptors were significantly up-regulated[37]. Therefore, MSG could cause changes in the structure of renal cells, an increase in the number of glomerular cells, inflammatory cells infiltration of the renal cortex and renal tubular cell edema, and ultimately leading to renal tubular degeneration.
The formation of reactive oxygen species (ROS) in the kidney is believed to be the main reason for MSG-induced kidney damage. Studies have shown that with MSG intake, the activity of some antioxidant enzymes (catalase, glutathione-S-transferase, superoxide dismutase, etc.) in the kidney is significantly reduced. In Wistar rats, after 9 months of free drinking water (2 mg/g/d), the expression of the oxidative stress marker protein heat shock protein homolog Hsc70 was up-regulated and glutathione-S-transferase P was down-regulated[38]. For instance, MSG may cause excessive production of free radicals, as well as reduce the activity of endogenous antioxidant enzymes to induce liver damage[39], and whether such pro-oxidant effect exists in the other organs should be examined in the future. Table 3 lists renal toxicities induced by MSG intake based on animal studies.
Cardiovascular diseases
-
Studies have shown that MSG can increase the incidence of cardiovascular diseases. Newborn rats receiving MSG (4 mg/g) have an obvious aortic vascular disease[40]. In Kumar & Bhandari's study, MSG administration (4 g/kg) was found to increase the oxidative stress of the heart as manifested by several heart disease markers such as lactate dehydrogenase, aspartate aminotransferase and alanine aminotransferase[41]. Meanwhile, intake of MSG (0.5−1.5 g/kg) caused myocardial infarction and fatal tachyarrhythmia in rats[42]. Extrapolation of the toxic doses in these studies and safe levels provided in dietary sources should be compared to provide conclusive evidence on potential toxicity of MSG.
Preclinical studies have shown that MSG also exerts a negative impact on fertility and embryonic development. As an excitatory neurotransmitter, glutamate can disrupt the HPA pathway, thereby reducing the level of sex hormones (including testosterone, follicle-stimulating hormone, and luteinizing hormone), which ultimately leads to abnormal sperm formation[43]. It has been found that there are a large number of glutamate receptors in the reproductive organs and sperm, posing the reproductive system to be very susceptible to excitatory damage caused by excessive glutamate ingestion. In Wistar rats, it was found that MSG (60 and 120 mg/kg) significantly reduced sperm motility in a dose-dependent manner, and increased sperm with abnormal morphology. Meanwhile, a certain toxic effect on the testis was also found, ultimately leading to male infertility[44]. In addition, high-dose intake of MSG can reduce sperm motility, stagnation of spermatogenesis, and edema. These changes may be related to the inhibition of superoxide dismutase, catalase and glutathione peroxidase activity in rodents after MSG ingestion[45].
MSG not only affects the male reproductive system, but also affects the female reproductive system. Current research showed that MSG affects the female reproductive system mainly through excitotoxicity and oxidative stress. MSG could cause arcuate lesions of the hypothalamus, resulting in a decrease in the release of catecholamine concurrent with a decrease in the luteinizing hormone releasing hormone. Therefore, MSG could cause hormone imbalance by disrupting the function of the hypothalamus-pituitary-ovarian axis[46]. Mondal et al. found that the levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH) and estradiol were significantly increased after oral administration of MSG (0.8, 1.6 and 2.4 mg/kg) to mating female rats for 30−40 d. Therefore, MSG may impair ovarian function by increasing the release of LH, FSH and estradiol and inhibiting follicular maturation[47]. In addition, in neonatal rats given MSG (subcutaneous injection of 4 g/kg every two days until the 10th day after birth), it was found that oocytes doubled, ovarian follicular cysts increased six times, and the number of primitive and atretic follicles also showed an increase[48]. In another study, it was found that when Wistar rats consumed MSG for 14 consecutive days, a decrease in the number of follicles versus an increase in atretic follicles is observed. Thus, it indicated that MSG is toxic to follicles and suppresses ovulation[49].
Cancer
-
The occurrence of tumors is the result of a comprehensive influence of multiple factors, not only the impact of the internal factors of the organism, but also the result of external environmental factors such as dietary factors. In a study of colorectal cancer (CRC), researchers found that the viability of cancer cells increased after 24 h of incubation with different concentrations of MSG, as well as an increase in the expression levels of adenomatous polyposis coli (APC) and beclin 1, which are involved in the development and progression of CRC. Therefore, it can be inferred that MSG may exert a promoting effect on the proliferation of CRC cells[6]. In a study of lung cancer, it was found that the glutamate receptor SLC1A1 was significantly up-regulated in tissue samples from patients with non-small cell lung cancer. Cancer cells actively uptake glutamate through SLC1A1 to promote the uptake of cystine through XC-, thereby promoting the biosynthesis of reduced glutathione (GSH) and alleviating the oxidative stress generated during the growth of cancer cells[50]. Newborn Wistar rats were injected subcutaneously with 400 mg/kg MSG before subcutaneous implantation of Walker-256 cells. The results showed that compared with the control group, the tumor volume in the MSG group was larger[51]. Moreover, newborn mice were given MSG (2 mg/kg) and azoxymethane (AOM), and the mRNA expression of insulin-like growth factor-1 (IGF-1) receptor in the MSG-AOM group was increased compared with mice given AOM only. These results suggested that MSG-treated mice are more sensitive to colon cancer induced by AOM[52]. This promotion of cancer may be related to the conversion of Gln to glutamate by glutaminase and further conversion to α-ketoglutarate through the tricarboxylic acid cycle. This reprogramming pathway plays a major role in ATP synthesis, maintaining redox balance, and regulating the energy expenditure of cancer cells[53].
Immune system
-
Studies have shown that MSG exerts a certain effect on the immune system. In vitro cell experiments showed that MSG significantly increased the frequency of chromosomal aberrations and sister chromatid exchange in human lymphocytes, and this change was dose-dependent. Therefore, MSG may have genotoxic effects on human peripheral blood lymphocytes[54]. It was also found that MSG has a dose-dependent effect on the cell viability of B cells, further to mediate apoptosis of memory cells and initial B cells by affecting the metabotropic glutamate receptor[55]. Likewise, primitive and memory lymphocytes express different glutamate receptors, which induce immune cell apoptosis[55]. In MSG-exposed rats (4 mg/g), it was found that the neuroendocrine-immune functions were altered[56]. Administration of MSG to mice (day 1, 2, 4, 6, 8, and 10 after birth) was found to ameliorate the defense mechanism of the mice against pathogenic microorganisms[57]. In another animal experiments, MSG (4 mg/g, i.p. for 6 d) appeared to inhibit the proliferation of thymocytes, which may be related to the promotion of cell apoptosis[58], or it may be associated with the increase of oxidative stress[59]. The natural killer cell cytotoxicity depression was correlated to the decreased numbers of large granular lymphocytes in MSG-treated mice[60].
Miscellaneous effects
-
In addition to the above-mentioned adverse effects, MSG could also result in asthma[61], allergic reactions and genetic toxicity. Zanfirescu et al. found that after 21 d of administration of MSG (300 mg/kg) in mice, the thermal nociception threshold was significantly reduced, which may be related to the increase in brain nitrate concentration[62]. After the neonatal rats were injected with MSG (4 mg/g), it was found that MSG had a certain effect on the regulation of rats hypothalamic center in the pituitary-thyroid axis[63]. It was also reported that MSG exhibits a certain effect on the cognition and hippocampal synapse of obese mice[64]. MSG could also cause endocrine dysfunction in mice, which may be related to the lack of Enk cells and fibers in the hypothalamus[65]. Studies have shown that neonatal MSG treatment (2 or 4 g/kg) could lastingly promote the spread of cortical depression in rats. Therefore, consumption of MSG in developing organisms requires careful monitoring and or whether such effects could occur from mothers feeding on MSG should be determined[66]. In addition, it reported that administration of MSG in the second or third trimester not only makes the offspring obese, but it has also been found to cause behavior defects in the offspring[67]. MSG could also induce apoptosis of thymocytes, which may be related to the oxidative stress induced by MSG in appetite[68]. Meanwhile, it has been found that gavage of MSG could accelerate the occurrence of Alzheimer’s disease in APP/PS1 mice[69]. Further, adult rats treated with MSG, showed behavioral deficits[70]. Demirkapu et al. reported that long-term administration of MSG (1.0 g/L) during the developmental period of rats could cause excessive excitement of the central nervous system (CNS) and lead to epilepsy[71]. Rats administered with high doses of MSG (3.0 and 3.5 mg/g) also affects motor coordination, and the number of Purkinje cells in the cerebellum is also significantly reduced[72]. Studies have shown that when rats consume MSG, pancreatic lysosomes have also undergone corresponding changes. The main manifestations include swelling of mitochondria, changes in zymogen particles, with the ultrastructure of pancreatic acinar cells revealing an increase in the number and size of autophagic vacuoles[73]. Ataseven et al. co-incubated different concentrations of MSG (250, 500, 1,000, 2,000, 4,000 and 8,000 μg/mL) and human lymphocytes in vitro, and found that MSG can cause DNA damage to human lymphocytes, chromosomal aberrations, sister chromatid exchange, and cell division after 1 h in vitro incubation. The frequency of aberrations that block micronuclei increases, suggesting that MSG is genetically toxic to human peripheral blood lymphocytes in vitro[54]. In addition, it was found that MSG can limit the adipogenicity potential of 3T3-L1 preadipocytes, and this may be related to the clonal expansion of mitosis[74]. In 1981, Allen & Baker reported for the first time that asthma occurred 12 h after eating in a Chinese restaurant[75] Subsequently, they reported that 14 of 32 patients had asthma attacks after ingesting MSG in 1987[76]. A double-blind, placebo-controlled, crossover study revealed that after 14 healthy men drank sugar-free soda containing MSG for 2 h, human subjects had a significant increase in headache and pericrania muscle tenderness[77]. In addition, it is closely related to the occurrence and progression of psychiatric symptoms[78]. A clinical study showed that in Chinese adults of normal weight, the intake of MSG may increase the risk of sleep disordered breathing[79]. Meanwhile, after analyzing the data of 1,227 subjects participating in the Jiangsu Nutrition Study, it was found that the intake of MSG may independently increase blood pressure[80]. MSG induced miscellaneous injuries are summarized in Table 4.
Table 4. MSG-induced miscellanious injuries.
Diseases Dosage/day Species Effects References Cardiovascular diseases 4 mg/g Newborn rats Resulted in typical adult life MetS and oxidative stress with significant increase in ET-1 and MMP-1 with aortic vasculopathy [41] 4 g/kg Neonatal Wistar rats Induced dyslipidemia and oxidative stress [42] I.v 1.5 g/kg Wistar rats Induced bradycardia [43] Fertility and fetal development 60 and 120 mg/kg Adult male rats Significant damage to the reproductive system [45] 6, 17.5 and 60 mg/kg Mature male Wistar rats Induced testicular toxicity and reduce the oxidative stress on testis tissues [46] S.c. 4 g/kg Male and female Wistar rats Male rats were more susceptible to induce metabolic alterations [47] 0.8, 1.6 and 2.4 mg/kg BW/day Female virgin albino rats Suppressed the female reproductive function by impairing the functions of ovary and uterus [48] S.c 4 g/kg Pregnant Wistar rats Decrease in oocyte count and increase the total primordial and atretic follicles [49] 0.2 g/kg Female Wistar rats Decrease in primary follicle count and increased in atretic follicle count [50] Cancer S.c 400 mg/kg Newborn male Wistar rats Increased tumor growth [51] S.c 2 mg/g The pregnant ICR mice Highly susceptible to AOM-induced colorectal carcinogenesis [52] Immune system Human lymphocytes 250, 500, 1,000, 2,000, 4,000 and 8,000 μg/mL Showed genotoxic to the human peripheral blood lymphocytes [55] 1−100 mM B cells Induced apoptosis in naïve and memory human B cells [56] I.p. 4 mg/g Adult male and female Sprague-Dawley rats Induced immune derangements [57] 2 mg/g C57BL/6J male mice Impaired the defense mechanism against invasion of pathogenic microbes [58] I.p.4 mg/g Male Wistar rats Triggered the thymocyte apoptosis [59] 4 mg/g − Induced thymocyte apoptosis [60] − Newborn mice Destructed the mouse arcuate nucleus [61] Miscellaneous ellaneous effects 300 mg/kg Male NMRI mice Induced hyperalgesia [63] 4 mg/g Newborn rats Affected the thyroid function [64] 2 mg/g Newborn mice Induced obesity and recognition memory deficits [65] − Mice Exhibited endocrine dysfunctions [66] 2 or 4 g/kg Male Wistar rat Influenced cortical excitability [67] − Rats Caused behavioral deficits [69] 4 mg/g Adult male Wistar rats Induced oxidative stress and apoptosis in thymocyte [60] 0.5% MSG and 1% MSG APPswe, PSEN1dE9-85Dbo/J transgenic mice and WT mice Accelerated AD-like pathophysiology in a
mouse model of AD[70] S.c 3 mg/g Pregnant Hannover-Wistar female rats Reduced locomotion [71] 1.0 g/L Wistar Albino rats Increased CNS excitability [72] 3.0 and 3.5 mg/g Wistar rats Decreased motor coordination and the estimated total number of Purkinje cell of rats [73] 0.05%, 0.1%, 0.2%
and 0.5%Male Wistar rats Induced pancreatic acinar cells injury [74] 250, 500, 1,000, 2,000, 4,000 and 8,000 μg/mL Human lymphocytes Induced genotoxicity [55] 100 μM, 250 μM, 2.5 mM and 25 mM 3T3-L1 preadipocytes cells Changes in cell proliferation and lipid accumulation [75] 2.5 g Two young women Induced asthma [76] − Subjects Induced asthma and make recognition difficult [77] 75 or 150 mg/kg 14 healthy men Induced headache and craniofacial pain [78] − − Induced chronic pain [79] − 1,227 Chinese subjects Increased the risk of SDB in Chinese adults [80] − 1,227 Chinese men and women Independent BP-increasing effects [81] -
This review highlights that MSG ingestion can be associated with obesity, NAFLD, neurotoxicity, renal toxicity, cardiovascular diseases and cancer in different models (including cell, animal, and clinical studies). Therefore, it is of value to identify approaches that can aid or prevent against the negative effects of MSG. Current research shows that some medicinal plants and nutraceuticals have a good protective effect on the diseases induced by MSG (Fig 4). Table 5 lists the main plants, natural products and specific mechanisms that exert protective effects as also discussed subsequently.
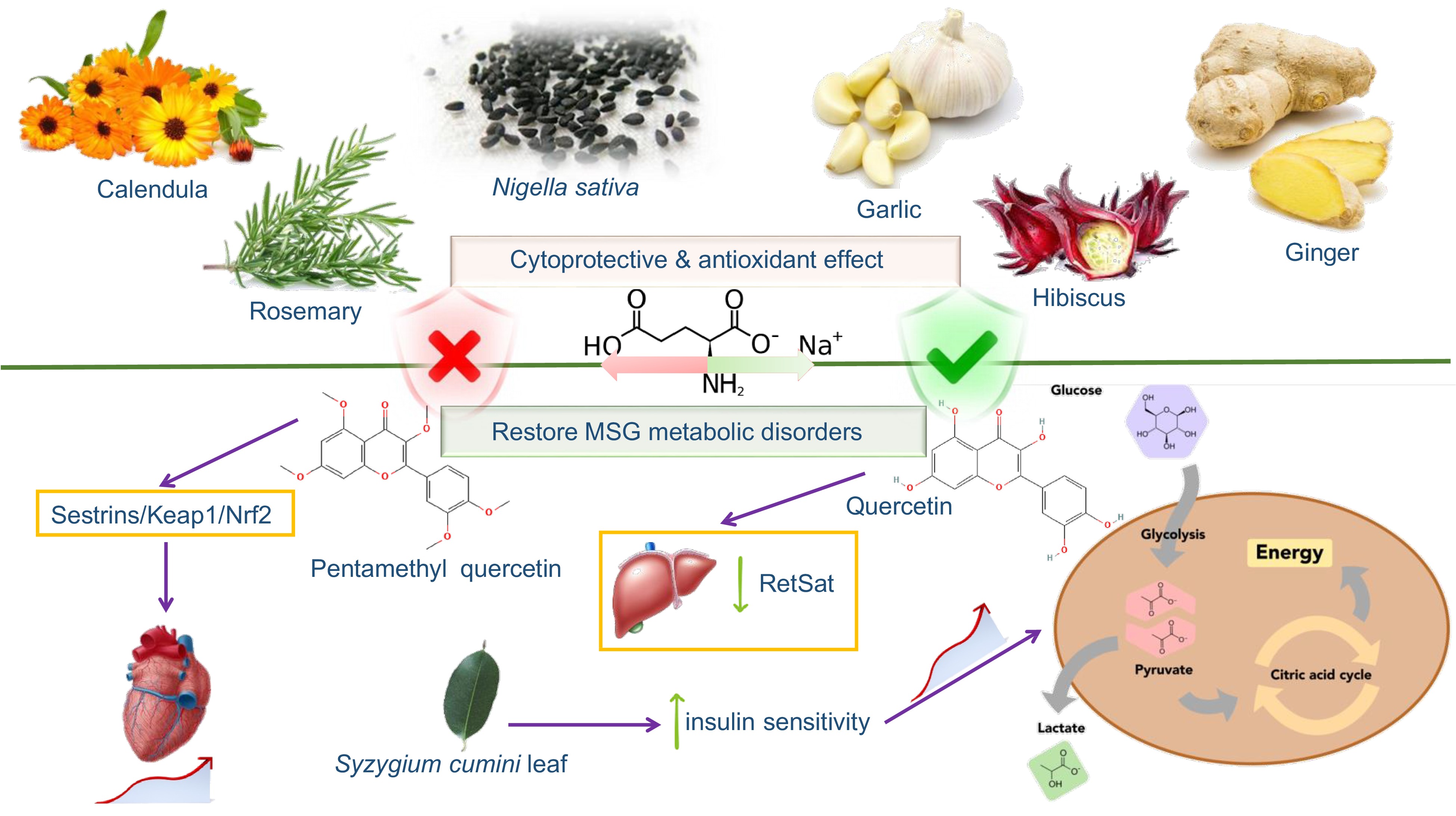
Figure 4.
Nutraceuticals and natural products protective effect against MSG toxic actions in human functions.
Table 5. Protective effects of some plants or natural products against various MSG negative health effects.
Target MSG-induced diseases Compound Dose or concentration Experimental model Effects References Metabolic syndrome Vitamin D 0.2 mcg/kg Female rats Suppressed body weight [82] Probiotics 109 CFU/kg Newborn rats Recovery of lipid metabolism and prevention of the obesity development [83] Pentamethylquercetin 5, 10 and 20 mg/kg Obese mice Ameliorated obesity phenotypes and improved metabolic disorders [84] Quercetin 5 mg/kg C57BL/6J mice Reduced the hypothalamus damage and the expression of liver RetSat [85] n-6/n-3 PUFAs n-6/n-3 fatty acid ratio of 1.2: 1.0 Female mice Prevented metabolic dysfunction [86] Quercetin 75 mg/kg Male Wistar rats Normalized serum lipid and glucose profile [87] (p-CIPhSe)2 10 mg/kg Newborn Wistar rats Reduced hepatotoxicity [89] Berberine 150 mg/kg Newborn rats Alleviated postnatal metabolic disorders associated vascular endothelial dysfunction [41] Polyphenol-rich extract of Syzygium cumini leaf 500 mg/kg Obese rats Improved peripheral insulin sensitivity [90] Nervous system Allium sativum powder 200 mg/kg Male Wistar albino rats Anti-oxidative stress and apoptosis [91] Calendula officinalis L. flowers 100 and 200 mg/kg Adult female Wistar rats Antioxidant and anti-inflammatory [92] Rosemary extract and/
or fluoxetine50 mg/kg Male Sprague-Dawley rats Antioxidant and anti-inflammatory [93] Kidney Zinc oxide nanoparticles − − Reduced the level of intracellular peroxidation by enhancing antioxidant capacity [95] Nigella sativa seeds 30 g/kg Male adult albino rats Rebalanced LPO and TAC, antioxidant and cytoprotective activities [96] Others Hibiscus sabdariffa extract 180 mg/kg Adult rats Reduced oxidative stress and cellular apoptosis [97] N-Acetylcysteine 500 μM C6 cells Protected C6 stellate cells [98] Metabolic syndrome
-
Studies have shown that vitamin D can effectively alleviate MSG-induced obesity[81]. Regular administration of probiotics to newborn rats could restore the lipid metabolism disorder and obesity caused by MSG[82]. Identification of exact microbial strain mediating these beneficial effects should be examined to be conclusive. Du et al. reported that pentamethylquercetin could improve cardiac remodeling in MSG-induced obesity of mice through the Sestrins/Keap1/Nrf2 signaling pathway[83]. In the study by Zhao et al., dietary quercetin was found to reduce the hypothalamus damage and reduce the expression of liver RetSat by regulating intestinal flora imbalance induced by MSG, thereby combating MSG-induced abdominal obesity[84]. Moreover, Baccharis dracunculifolia methanol extract[85], Cedrus deodara extract[86] and (p-CIPhSe)2[87] showed good resistance to MSG-induced obesity. Identification of the active agent in these dietary sources or functional foods such as Cedrus deodara should be thoroughly investigated and the underlying action mechanisms understood.
In addition, Abo et al. showed that berberine alkaloid alleviated MSG-induced postnatal metabolic disorders associated vascular endothelial dysfunction in newborn rats[40]. Bezafibrate, a fibrate drug used as a lipid-lowering agent to treat hyperlipidemia was found to effectively improve the metabolic syndrome induced by MSG in a mouse model of NASH[88]. Polyphenol-rich extract of Syzygium cumini leaf was reported to likewise improve peripheral insulin sensitivity by regulating β-cell insulin release, in obese MSG-induced metabolic syndrome rats[89].
Nervous system
-
A study suggested that Amphora coffeaeformis algae has a potential neuroprotective effect on the neurotoxicity induced by MSG in adult rats[5]. Ginger and propolis exerted similar protective effects, though with no evidence of the underlying chemical profile[33]. In addition, Allium sativum powder could improve MSG-induced neurotoxicity by alleviating the proliferation and apoptosis of glial cells in rat brain tissues induced by oxidative stress[90]. Other reported nutraceuticals to exert similar effect include the extracts of ginger (Zingiber officinale) root[91], quercetin[92] and AMP-activated protein kinase (AMPK) activator Pioglitazone[93].
Kidney
-
Zanuzo et al. reported that the proper physical exercise and vitamin D supplementation could increase the thickness of the renal cortex and reduce the degeneration of the renal tubules, thereby preventing kidney damage[94]. Zinc oxide nanoparticles reduced the level of intracellular peroxidation by enhancing cellular antioxidant capacity, thereby reducing the kidney damage caused by MSG in rats. In addition, Nigella sativa L. seeds have also been found to reduce MSG-induced testicular damage through antioxidant and cytoprotective activities[95]. Whether such effects can be attributed to the major phytochemical thymoquinone in nigella seed has yet to be confirmed.
Miscellaneous
-
It was reported that Hibiscus sabdariffa or roselle extract could reverse MSG-induced testicular toxicity by reducing oxidative stress and cellular apoptosis in adult rats[96]. Park et al. found that N-acetylcysteine exerts a protective effect on the toxicity of C6 astrocytes induced by MSG, and this may be related to the protection of C6 stellate cells from MSG-induced oxidative stress, mitochondrial dysfunction and endoplasmic reticulum stress[97]. Studies showed that exercise could help both developing and adult brains against the harmful effects of MSG[98], this notion was supported by Araujo et al.[99]. Studies have found that (p-CIPhSe)2 can reverse MSG high glucose metabolism and inhibit mitochondrial malfunction in rats[100].
-
Being an essential amino acid for the human body, MSG participates in various metabolic reactions. Currently, there are no strict regulations on the daily intake of MSG. The FDA stipulates that MSG should be labeled on the outer packaging of a product, but in fact, MSG is also found in natural ingredients such as soy and yeast extracts. Therefore, this makes it difficult to define the extract amount of consumers’ daily ingestion. Research showed that China is the country with the highest consumption of MSG worldwide. Asia has become the region with the highest consumption of MSG due to the increasing demand for MSG in Indonesia, Thailand and Vietnam. However, MSG is banned in America, Canada and Mexico due to rising obesity rates.
Generally, relevant regulations in the food field consider additives to be safe and harmless to the human body. However, an increasing number of preclinical and clinical studies have raised doubts about MSG safety. Normal doses of MSG have important functions in the human body, but long-term high concentrations of MSG may have adverse effects on the human body, with multiple effects on organs and systems. We speculated that the main reason for the contradiction lies in the misuse or overuse of MSG. Another reason is that with the continuous development of society, humans have stricter requirements on the taste of food, so more condiments are added to encompass the requirements, which leads to people ingesting more MSG, increasing the risk of side-effects. The third possibility is that, due to the limitation of technical means or the short observation time, it was mistakenly hypothesized that MSG is not harmful to the human body, with no evident restriction on the use of MSG. Together, more scientifically rigorous, larger-scale preclinical and clinical studies need to be carried out. In addition, investigating another umami enhancer may also be a safe alternative for human health.
-
In conclusion, MSG, as a food additive, produces a special umami taste by activating TAS1R1-TAS1R3 on the tongue. It is favored by people worldwide, and as the use of MSG has increased, its safety has been controversial. Research results in recent years have shown that excessive intake of MSG will produce a series of adverse effects on the human body, including obesity, metabolic syndrome, kidney, immune, reproductive, nervous, and cardiovascular and cerebrovascular system damage. Therefore, it is necessary to strengthen publicity and education on the harmful effects of MSG overdose on the human body and raise people’s awareness. On the other hand, more rigorous human clinical experimental studies on MSG also need to be carried out. In cell and animal models, MSG overconsumption related health effects have been sufficiently confirmed; however, a lack of clinical studies significantly limits the reliability of the obtained information. With the advances in metabolomics and other omics, better monitoring of MSG metabolism can be also achieved. With the development of multi-omics and bioinformatics, intestinal flora has been confirmed to be closely related to human digestion, absorption, metabolism, immunity and other functions. Such in depth studies of the biological and functional activities of the metabolites derived following ingestion will consolidate the understanding of the link between the health effects and the intake of MSG.
This study was supported by National Natural Science Foundation of China (32072212).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Yang L, Gao Y, Gong J, Peng L, El-Seedi HR, et al. 2023. A multifaceted review of monosodium glutamate effects on human health and its natural remedies. Food Materials Research 3:16 doi: 10.48130/FMR-2023-0016
A multifaceted review of monosodium glutamate effects on human health and its natural remedies
- Received: 10 November 2022
- Accepted: 29 March 2023
- Published online: 21 July 2023
Abstract: Monosodium glutamate is a non-essential amino acid in the human body that has been widely used as a food additive since 1909 due to its unique umami taste. Typical food containing monosodium glutamate include tomatoes, yeast extracts, fermented soy products, and cheese. After entering the human body, it is mainly absorbed in the small intestine by passive diffusion. Subsequent binding to TAS1R1-TAS1R3 promotes the release of intracellular calcium that ultimately activates the brain’s taste center, recognized as an umami taste of monosodium glutamate. However, more and more preclinical and clinical data indicates that monosodium glutamate exerts adverse effects on humans, including metabolic syndrome, neurotoxicity, renal toxicity, cardiovascular disease, infertility and fetal underdevelopment, cancer, immune malfunction and so on. In addition, it has been found that medicinal plants and nutraceuticals could mitigate or treat the adverse effects of monosodium glutamate adverse. Taken together, we hope to raise the correct awareness of monosodium glutamate and improve the understanding of monosodium glutamate quality approaches to maximize their use.
-
Key words:
- Monosodium glutamate /
- Health /
- Safety /
- Toxicity /
- Natural products