-
Tea is produced from the tender leaves of tea plant (Camellia sinensis) and is one of the most widely consumed beverages worldwide. Although aroma compounds account for less than 0.03% of the tea leaf dry weight, they substantially affect consumer preferences for tea beverages[1]. Indole is a significant aroma-enhancing component in tea. Its biosynthesis and contribution to abiotic stress responses during the processing of tea leaves have been investigated. A gas chromatography/mass spectrometry-olfactometry analysis revealed that indole is a significant contributor to the strong and natural floral aroma of oolong tea. Indole accumulates during the processing of oolong tea[2]. The genes responsible for indole synthesis in tea, including genes encoding the tryptophan synthase α-subunit (TSA) and three tryptophan synthase β-subunits (TSBs), have been reported[3]. Of these genes, CsTSB2 is highly expressed during the turn-over stage of oolong tea production. Furthermore, low-temperature stress (15 °C) and mechanical damage synergistically affect indole synthesis, possibly via the increased expression of CsTSB2[4].
In addition to influencing tea aromas, indole has other biological functions in plants. For example, indole is an important metabolite for plant responses to external stresses and is involved in plant defenses. Indole synthesis and release is induced by insect infestations of diverse plants, including peanut, rice, and maize[5−7]. In maize, indole is released before terpenoids, suggesting indole may rapidly initiate defense response-related signaling pathways in plants following an exposure to stress[5]. In a previous study, indole was observed to activate defense responses in unaffected tissues or the tissues of neighboring plants following an infestation by herbivores[8]. Economically valuable tea plants are typically susceptible to a wide variety of insect pests. More specifically, the infestation of tea plant by tea geometrid (Ectropis grisescens Warren), tea leafhopper and Myllocerinus aurolineatus Voss can induce indole formation and release[9]. Accordingly, indole appears to be a common volatile produced by tea plant to limit the damage caused by insect pests. Furthermore, indole can induce systemic defenses in neighboring healthy tea plant, thereby enhancing insect resistance[10,11]. However, the pest-induced release of indole and the associated regulatory mechanisms are unclear, which has restricted the utility of indole for controlling pests in tea gardens. Thus, further characterizing the insect resistance-related substances in tea plants may be beneficial for developing environmentally safe methods for protecting tea plant. In this study, we systematically analyzed the pest-induced indole release pattern using a real-time on-line absorption-desorption device. We also explored the mechanism underlying the regular release of indole by tea plant, with potential implications for improving pest control measures. The study results have enhanced our understanding of indole biosynthesis and release patterns in tea plant, while also providing a candidate target relevant to preventing pest infestations of tea gardens.
-
Tea and insect samples were collected from the Yingde Tea Experimental Station of the Tea Research Institute, Guangdong Academy of Agricultural Sciences (China). The variety of tea samples was C. sinensis 'Jinxuan'. The common chewing pests were tea geometrid and cultured in our laboratory. Nicotiana benthamiana were grown under 22 °C, 60% humidity and 16-h light/8-h dark photoperiod. Vespa velutina nigrithorax Buysson was one of the most common wasp species and kept in the tea plantation of the Yingde Tea Experimental Station of the Tea Research Institute, Guangdong Academy of Agricultural Sciences (China).
Evaluation of the anti-insect property of indole
Exogenous indole feeding treatment (direct method)
-
The method was as per a previous reference[12]. Before the treatments, the tea geometrids (3rd instar) were stopped feeding for 12 h. Indole solution was added to tea powder (10 g). Low concentration treatment: 70 μg indole dissolved in 5 mL water containing 1% (v/v) ethanol. While the high concentration treatment contained 350 μg indole. Control with 5 mL water containing 1% (v/v) ethanol. Tea geometrids were weighed every 24 h. Each treatment contained 25 replicates, which contained one tea geometrid.
Indole fumigation treatment (indirect method)
-
The method was as per a previous reference[12]. Before the treatments, the tea geometrids (3rd instar) were stopped feeding for 12 h. Treatment groups included 2 or 20 mM indole which was dissolved in dichloromethane. The concentration of indole fumigation treatment was based on the release of indole in tea leaves. Tea branches were fumigated by 500 µL of indole solution to cotton balls for 12 h. The samples were treated continuously for 48 h. The control group was fumigated with dichloromethane. After fumigation, the branches were fed to tea geometrids. Tea geometrids were weighed every 24 h. Each treatment contained 25 replicates, which contained one tea geometrid.
Effect of exogenous indole on the behavior of enemy of pests (indirect method)
-
Vespa velutina nigrithorax Buysson was a common natural enemy of tea plant pests and was selected to explore the attraction of indole to natural enemies of pests. The channels of the Y-tube were placed separately for the indole treatment and control. The two concentration treatments of indole were 2 and 20 mM. Dichloromethane treatment was set as the control. It was monitored for 15 d, and the recording was started at 11:00 AM every day, and the number of Vespa velutina nigrithorax Buysson were recorded once every 15 min.
Analysis of indole emission in tea branches attacked by tea geometrid under normal photoperiod conditions
-
Indole emission was analyzed by real time collection–programmable temperature vaporizer-gas chromatography-mass spectrometry (PTV-GC-MS), which could automatically collect, desorb, and analyze volatiles[12]. Tea branches were pruned to one bud and three leaves, and left to stand for 24 h after pruning. The glass container was put into eight tea branches and five tea geometrids (3rd instar). Volatiles were collected and analyzed continuously for 3 d under 25 °C, 16-h light/8-h dark photoperiod, and 60 % humidity. Volatiles were analyzed by gas chromatography-mass spectrometry (GC-MS, Shimadzu Corporation, Kyoto, Japan) detection combined with SUPELCOWAX 10 column (Supelco Inc., 30 m × 0.25 mm × 0.25 μm). The conditions of the GC-MS assay were the same as the previous reference[12].
Analysis of indole emission in tea branches subjected to continuous mechanical damage under different light conditions
-
Thermal desorption gas chromatography mass spectrometry (TDU-GC–MS) was used to analyze indole emission[12]. Tea branches were pruned to one bud and three leaves, and left to stand for 24 h after pruning. Each group contained five or eight replicates. Five tea branches were put into the glass container as one replicate. The light conditions were set as follows: (1) Tea branches were cultivated under normal photoperiod (16-h light/8-h dark); (2) Tea branches were cultivated under continuous light; (3) Tea branches were cultivated under continuous dark. Other conditions were the same. Volatiles were collected by PTFE tubes which were replaced by after continuous collection for 6 h, and two holes were punched into each leaf using a 3 mm caliber perforator. After collection, volatiles were analyzed by Agilent 8890/7000D triple quadrupole GC/MS system with a SUPELCOWAX 10 column (Supelco Inc., 30 m × 0.25 mm × 0.25 μm). The conditions of the GC-MS assay were the same as the previous study[12].
Analysis of endogenous indole in tea leaves subjected to continuous mechanical damage under normal light conditions
-
Endogenous indole was extracted by organic solvents and analyzed by GC-MS. Tea branches (one bud and three leaves) cultivated under a normal photoperiod (16-h light/8-h dark) were punched once every 3 h with a perforator. Tea leaves were collected every 6 h and continuously collected for 72 h. Each group contains three replicates. Throughout the experiment, all samples were treated under normal photoperiod. Method of aroma extraction was referred as per a previous reference[12]. Tea powders (300 mg) were extracted by 1.8 mL dichloromethane containing 5 nmol ethyl n-decanoate as an internal standard. After dewatering and concentrating, the aromas were analyzed with GC-MS as described above.
Analysis of the expression level of genes
-
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to study the expression level of genes. Total RNA of samples was extracted by Quick RNA isolation Kit. The primers used for qRT-PCR were shown in Supplemental Table S1. iTaqTM Universal SYBR Green Supermix were used in the qRT-PCR analysis carried out on Roche LightCycle 480 (Roche Applied Science, Mannheim, Germany). Encoding elongation factor 1 (CsEF1-α) (Genebank No. KA280301.1) was used as an internal reference gene. More information can be referred from a previous reference[12].
Yeast two-hybrid assays
-
The recombinant plasmids AD-CsTSA (full-length ORF sequence of CsTSA fused into pGADT7 vector) and BD-CsTSB1, BD-CsTSB2, and BD-CsTSB3 (full-length ORF sequence of CsTSB1, CsTSB2, and BD-CsTSB3 fused into pGBKT7 vector) were co-transformed into the yeast strain AH109. Primer sequences are shown in Supplemental Table S2. The yeast cells were grown on DDO medium (SD/-Leu/-Trp) for 3 d. The grown single colonies were transferred to selective QDO medium (SD/-Leu/-Trp/-His/-Ade), and possible interactions between CsTSB2 and CsTSA were detected by X-gal staining.
Firefly luciferase complementary imaging assays
-
The firefly luciferase complementary imaging (LCI) refers to previous literature with minor modifications[13]. The ORF sequences of CsTSB2 and CsTSA were cloned into luciferase (CLuc) and luciferase (NLuc) vectors to form recombinant plasmids CsTSA-CLuc and CsTSB2-NLuc. The recombinant plasmids were transfected into Agrobacterium tumefaciens GV3101-pSoup and co-infected into tobacco leaves. After 36 h of culture, the lower epidermis of the leaves was sprayed with fluorescein (100 μM) and kept in the dark for 5 min to extinguish chlorophyll autofluorescence. Images were taken by a CCD imaging apparatus (Tanon 5200, USA). Primer sequences are shown in Supplemental Table S2.
Co-immunoprecipitation (Co-IP) assays
-
The Co-IP assays was carried out as per a previously published method[14]. The constructed CsTSA-GFP and CsTSB2-FLAG vectors were transferred into Agrobacterium tumefaciens GV3101-pSoup and co-infected into tobacco leaves. After 36 h, the tobacco leaves were collected and subjected to protein extraction. The protein supernatant obtained was added to anti-FLAG beads and incubated at 4 °C for 3 h. The proteins were collected and washed three times. Immunoprecipitated proteins were detected by Western Blotting with anti-GFP and anti-FLAG antibodies. The primer sequences are shown in Supplemental Table S2.
Statistical analysis
-
The significance was determined by SPSS 18.0 software based on paired and independent samples t-tests. All data are expressed as the mean ± standard deviation (SD).
-
The production of volatiles is often a part of plant defenses against stress. Earlier research showed that exogenous indole can activate calcium ion and jasmonic acid signaling pathways to promote the formation of defense-related metabolites[11]. Hence, indole may indirectly enhance tea plant defenses through signal transduction pathways. In this study, we comprehensively analyzed the effects of volatile indole produced by tea plant. The addition of exogenous indole to tea powder for tea geometrids (i.e., common chewing pest of tea plants) revealed that exogenous indole does not directly affect tea geometrids growth and development (Fig. 1a). However, growth and development of tea geometrids fed with tea branches fumigated using indole were significantly inhibited (Fig. 1b). These results suggest that indole may mediate signal transduction and regulate defense responses in plants. A previous study involving maize plants showed that indole can attract the enemies of pests and promote the defenses of neighboring plants[5]. Therefore, we also explored whether indole is an attractant for the enemies of pests that infest tea plant. Exogenous indole significantly attracted Vespa velutina nigrithorax Buysson, which is an enemy of tea plant pests (Fig. 1c). Considered together, these findings indicate volatile indole may indirectly enhance pest resistance via plant signal transduction pathways and the attraction of natural enemies.
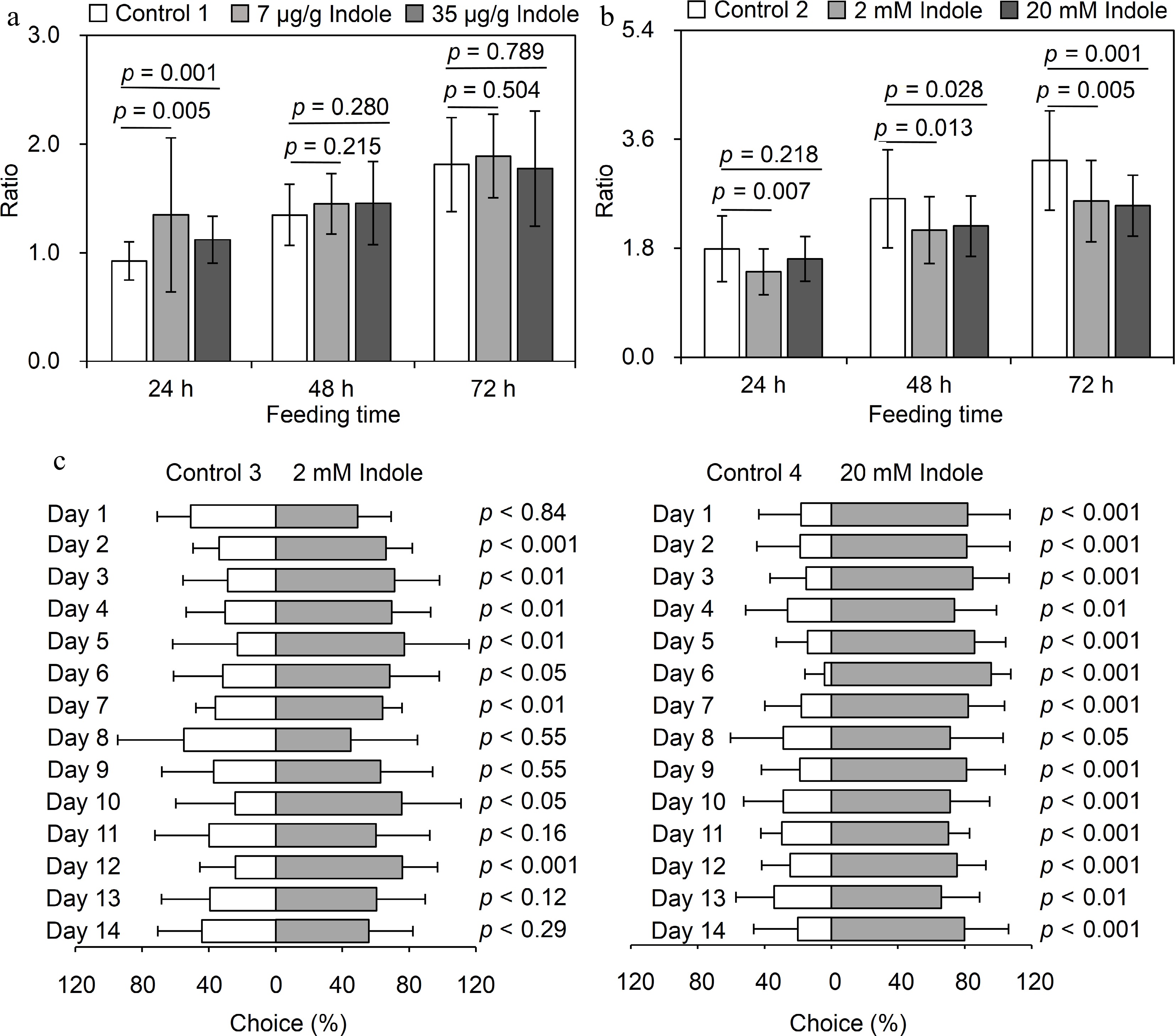
Figure 1.
Evaluation of anti-insect property of indole. (a) Exploration of direct defense. Control 1: water containing 1% ethanol was added to tea powder; Low concentration treatment: 7 μg/g indole (containing 1% ethanol) was added to tea powder; High concentration of treatment: 35 μg/g indole (containing 1% ethanol) was added to tea powder. (b) Exploration of signal transduction. Control 2: tea branches were fumigated with dichloromethane; Low concentration treatment: tea branches were fumigated with 2 mM indole dissolved in dichloromethane; High concentration treatment: tea branches were fumigated with 20 mM indole dissolved in dichloromethane. Data are expressed as mean ± SD (n = 25). Significance was indicated between indole treatment and its corresponding control (p ≤ 0.05). (a, b): The ratio in the y axis means the weight of insects after feeding treatment (24, 48 and 72 h) to the weight of insects before feeding treatment (0 h). (c) Exploration of attracting natural enemy. Control 3 and Control 4, dichloromethane; Low concentration: 2 mM indole dissolved in dichloromethane; High concentration treatment: 20 mM indole dissolved in dichloromethane. Each treatment had 14 biological replicates and 12 technical replicates per replicate, respectively.
During their long-term co-evolution, plants and herbivorous insects developed distinct defense strategies. In response to an attack by herbivores, plants activate their defenses via the induction of various morphological changes, molecular mechanisms, and metabolic pathways. The extensive and dynamic defense mechanisms of plants result in the constitutive or external stress-induced synthesis of defense-related compounds[15]. Herbivore-induced plant volatiles (HIPVs) are specialized chemical messengers that are released when plants are damaged by herbivores. More specifically, HIPVs are released into the atmosphere by most parts of plant tissue[15]. On the basis of the species, developmental stages, and conditions of the plants and herbivores, different defense signaling pathways are activated and different HIPVs are produced[16]. These HIPVs play an important role in plant defenses because they can directly inhibit feeding by herbivorous insects, while also attracting the natural enemies of pests and activating intra- or inter-plant signaling pathways[17−19]. For example, glycosylation of 3-hexenol can directly protect plants from pests[20]. Behavioral studies showed that the terpenoids that can attract the natural enemies of plant pests include β-ocimene, linalool, DMNT (4,8-dimethyl-1,3,7-nonatriene), and β-caryophyllene[21,22]. In addition, 3-hexene ethyl ester was recently used to attract the enemies of M. aurolineatus and adult E. grisescens[23]. Another recent study confirmed HIPVs also mediate the defense responses in the undamaged tissues of plants attacked by herbivores or in neighboring healthy plants[24].
The perception of HIPVs by plants results in the induction of a series of defense responses, including changes in the membrane potential, activation of the Ca2+ signaling pathway, synthesis of plant hormones, and up-regulation of defense gene expression, which ultimately lead to significant increases in defense-related metabolites[24]. For instance, 3-hexene ethyl ester and indole treatments significantly activate the Ca2+ signaling pathway and the production of jasmonic acid to induce the biosynthesis of defense-related benzoxazinoids, which protect maize plants from herbivores[25]. In tea plants, an indole pre-treatment promotes the formation of defense-related plant hormones (e.g., OPDA, JA, JA-IIe and SA) and others metabolites (e.g., flavonoids, phenolamines, phenolic acids, and purine alkaloids)[11]. The fumigation of plants with indole reportedly restricts the accumulation of rutin and protects plants against many insect species, but it increases the sensitivity to Spodoptera litura[25]. Furthermore, indole-3-glycerol phosphate lyase catalyzes the conversion of volatile indoles to non-volatile benzoxazinones, which have gastrotoxic effects on insects[26]. As a pest-induced volatile, indole can protect model plants, such as rice and maize, from herbivores[5,25]. In the current study, indole released by tea plants also had indirect defense activities (Fig. 1).
Indole release by tea plants following an insect infestation is greater during the day than at night
-
Previous studies showed that insect infestations and mechanical damages can induce indole formation and release in tea plants[4]. However, the indole release pattern in response to a long-term continuous insect infestation and mechanical damage remains unknown. In this study, the long-term continuous analysis of HIPVs revealed that the release of indole following a tea geometrid infestation gradually increased in daytime and slowly decreased at night under normal photoperiod conditions (Fig. 2). In addition, the overall amount of indole released increased as the duration of the tea geometrid infestation increased, suggesting that the behavior of the insects and the severity of the infestation may be key factors influencing the amount of indole released. Currently, it is impossible to accurately model the frequency and intensity of insect feeding on leaves. To exclude the influence of insect behavior, we used a hole-puncher to uniformly damage tea leaves (i.e., to simulate the mechanical damage due to insect feeding) prior to examining the indole release pattern under normal photoperiod conditions or under continuous light or darkness. The results showed that the amount of indole released was greatest under light (Fig. 3). Hence, light may be one of the critical factors influencing indole release.
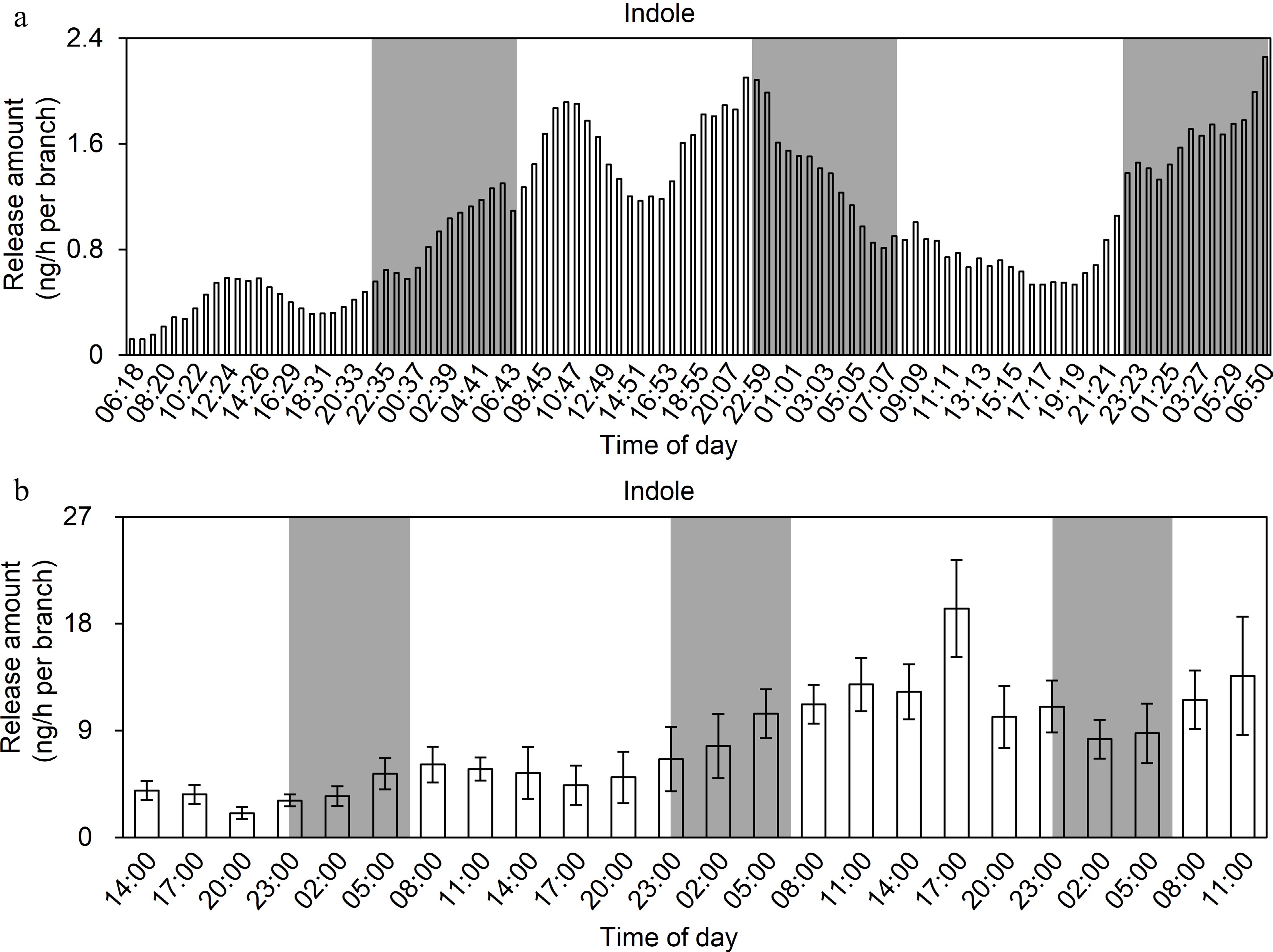
Figure 2.
Analysis of the emission pattern of indole in tea leaves under insect attack. (a) Real-time online analysis. Eight tea branches were attacked by five tea geometrids (3rd instar) in this experiment. Continuous monitoring with real-time online collection and analysis instrument. White background represents daytime and gray background represents night. (b) Analysis of TDU-GC–MS. Four tea branches were attacked by four tea geometrids (3rd instar) in this experiment. The volatiles were collected on PTFE columns and the columns were changed every 3 h. Data are expressed as mean ± SD (n = 8). White and gray background represents day and night, respectively.
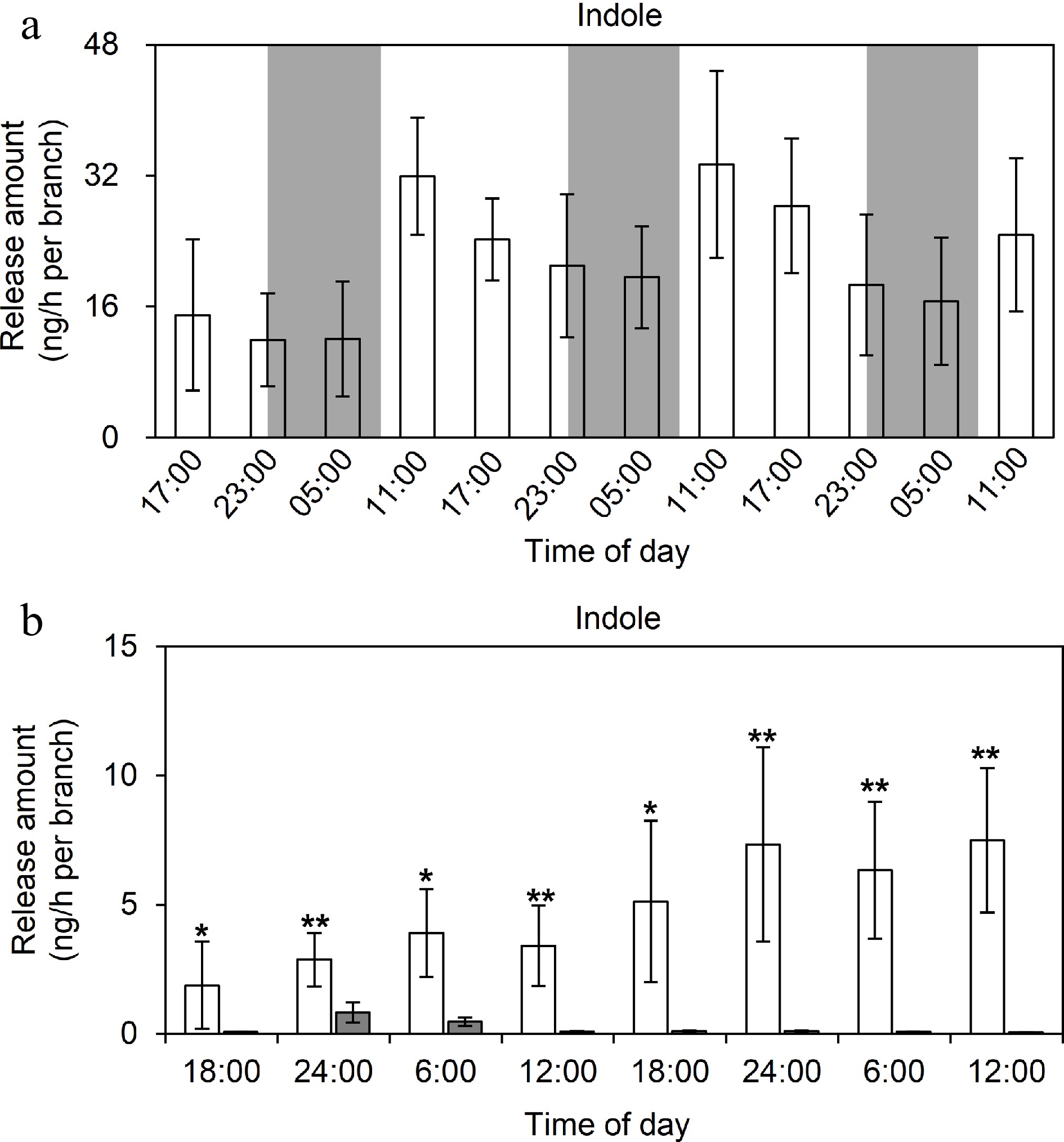
Figure 3.
TDU-GC-MS analysis of indole emission patterns under different light conditions. (a) The pattern of indole emission under normal photoperiods. Eight replicates were performed, and three tea branches were placed in each replicate, and the columns were changed every 6 h and two holes were punched into each leaf using a 3 mm caliber perforator. Data are expressed as mean ± SD (n = 8). White and gray background separately represents day and night, respectively. (b) The pattern of indole emission under continuous light or continuous dark. Five replicates were performed, five tea branches were placed in each replicate, and the columns were changed every 6 h and two holes were punched into each leaf using a 3 mm caliber perforator. Data are expressed as mean ± SD (n = 5). White and gray background represents continuous light and continuous dark, respectively. * and ** indicate significant differences between two treatments at the same time-point (p ≤ 0.05 and p ≤ 0.01, respectively).
Plants emit many terpenoids compounds, fatty acid derivatives, and benzene/phenylpropane derivatives[27]. The release of HIPVs (i.e., timing and amount) varies depending on the species, organ, developmental stage, and environmental factors[27]. The volatile mixtures produced by plants in response to different organisms vary depending on the time of day, thereby optimizing defenses, pollination, and reproduction. In general, terpenoid compounds, such as monoterpenes (linalool), sesquiterpenes [(E)-β-farnesene, (E)-(E)-α-bergamotene and α-duprazianene], and diterpenes [DMNT, TMTT (4,8,12-trimethyl-1,3,7,11-tridecatetraene), and isoprene], are released much more in the daytime than at night[10,28−30]. However, the timing of the synthesis and release of fatty acid-derived green leaf volatiles (GLVs) depends on the feeding patterns of the insect pests[31]. For example, after tobacco plants are attacked by Heliothis virescens, Helicoverpa zea, and Manduca sexta, (Z)-3-hexenyl acetate accumulates mainly at night. In contrast, black poplar trees attacked by Lymantria dispar release a large amount of GLVs during the day[32,33]. Benzene/phenylpropane compounds (e.g., methyl benzoate and benzyl alcohol) are the main plant floral volatiles. Insect-pollinated plants, such as rose, snapdragon, tobacco, and petunia, release benzene/phenylpropanoid volatiles at specific times during the day or night to attract pollinators and herbivores[34−36]. In addition, when non-floral plant tissues are attacked by insects, benzene/phenylpropane compounds are released to recruit the natural enemies of the insects[10,33].
Previous studies on the regular release of HIPVs mainly focused on model plants, such as Phaseolus lunatus, Petunia hybrida, and N. benthamiana, with relatively few studies on horticultural plants[35,37−38]. The rich and unique volatiles produced by tea plants influence the aromatic quality of the tea products[11], while also modulating tea plant defense responses to herbivores. Accordingly, tea plants have been used for analyzing the volatile organic compounds in non-model plants. After tea plants are attacked by herbivores, their leaves can release a variety of volatile compounds; the production of most of these compounds is induced by common pests. More than 50 volatile compounds may be released by tea plants following a tea geometrid infestation, of which 40 are also released after an attack by M. aurolineatus, including (E,E)-α-farnesene, ocimene, benzyl nitrile, (Z)-3-hexenyl acetate, nerolidol, indole, and DMNT[39]. However, the amounts of volatiles released as well as the time of the release vary depending on the phytophagous insect species, light conditions, and internal biological clock of the plants. For example, linalool, (E,E)-α-farnesene, and benzyl nitrile, which are released in response to an attack by M. aurolineatus, are mainly emitted at midday, whereas 1,3,8-p-menthatriene and (E)-β-ocimene emission is greatest at night, with the amount of the compound released increasing as the density of the insect population increases[39]. When Populus nigra is attacked by L. dispar, the emission of indole, which is a benzene/phenylpropane volatile compound, is 2- to 3-folds greater during the day than at night[33]. Other studies showed that insect pest infestations cause indole levels in plants to increase rapidly to peak levels and then gradually decrease as the infestation continues[5,10,39]. In the current study, a simulated insect infestation of tea branches resulted in more indole being released from leaves under light than in darkness, although the indole release pattern fluctuated somewhat (Figs. 2 & 3). Thus, indole synthesis and release may be regulated by light and insect feeding habits. The emitted indole also indirectly protected tea plants against insect pests (Fig. 1). Its regular release (i.e., high during the day and low at night) may be optimal for its biological function. Plant-derived defense-related substances may be useful for developing eco-friendly and sustainable pest control measures that can minimize the application of chemical pesticides.
The regular emission of indole is correlated with the biosynthesis involving CsTSB2, which combines with CsTSA to form a functional protein complex
-
To explore the factors influencing the indole release pattern of tea plants, the study analyzed the changes in the expression of indole synthesis-related genes. Mechanical damage significantly induced CsTSB2 expression, with a gradual increase during the day and a decrease at night, depending on the extent of the damage (Fig. 4). This expression trend was similar to the changes in the endogenous content and release of indole, implying CsTSB2 expression may be the main factor modulating indole production and release. A previous study indicated that CsTSA synthesizes indole from IGP in a process that requires CsTSB2, which is non-functional in the absence of CsTSA[3]. These two proteins may interact to form tetramers; however, whether these proteins actually form a protein complex to perform their functions remained untested. Therefore, in this study, the interaction between CsTSA and CsTSB2 was assessed, and only the yeast cells co-transformed with AD-CsTSA and BD-CsTSB2 grew normally on the QDO yeast selective medium (Fig. 5a). Moreover, the colony color change on the medium supplemented with X-gal reflected the interaction between CsTSA and CsTSB2 in yeast. The interaction between CsTSA and CsTSB2 was verified by conducting LCI and Co-IP assays. In the LCI assay, a strong fluorescent signal was detected in the positive control and the tobacco cells co-transformed with CsTSA-CLuc and CsTSB2-NLuc, indicative of the interaction between CsTSA and CsTSB2 (Fig. 5b). In the Co-IP assay, CsTSB2-FLAG was able to immunoprecipitate CsTSA-GFP, whereas CsTSA-GFP bands were undetectable in the control (Fig. 5c). Considered together, these results suggest that CsTSA interacts with CsTSB2 both in vitro and in vivo.
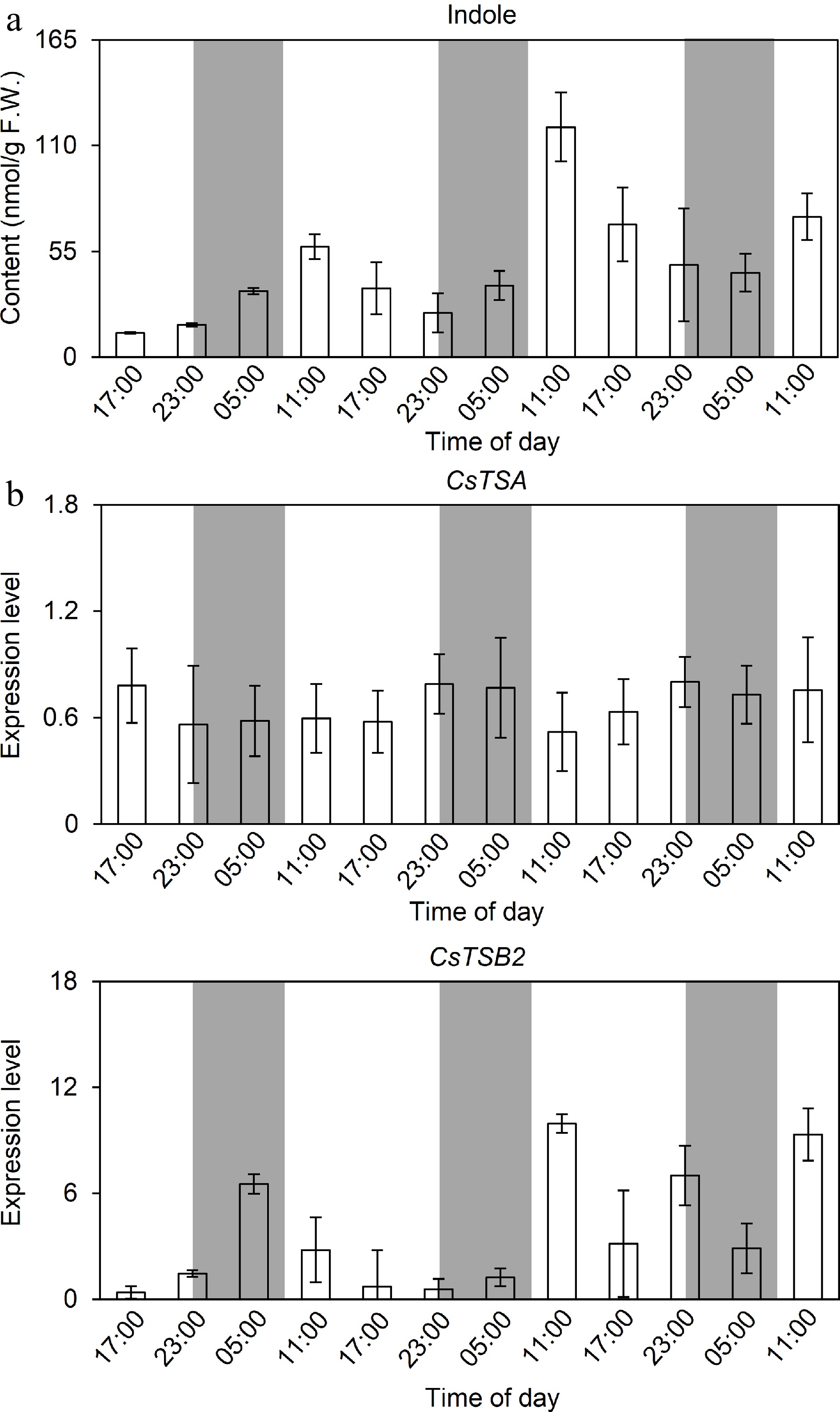
Figure 4.
Variation of endogenous indole content and expression level of its structural genes under mechanical damage. (a) Variation of endogenous indole content under continuous mechanical damage. (b) Variation of expression level of synthesizing genes under mechanical damage. Samples were collected at 6 h intervals, and five replicates with three tea branches placed in each replicate were performed. Each leaf was punched once/3 h per leaf by a 3 mm caliber punch. Data are expressed as mean ± SD (n = 5). White and gray background represents day and night, respectively.
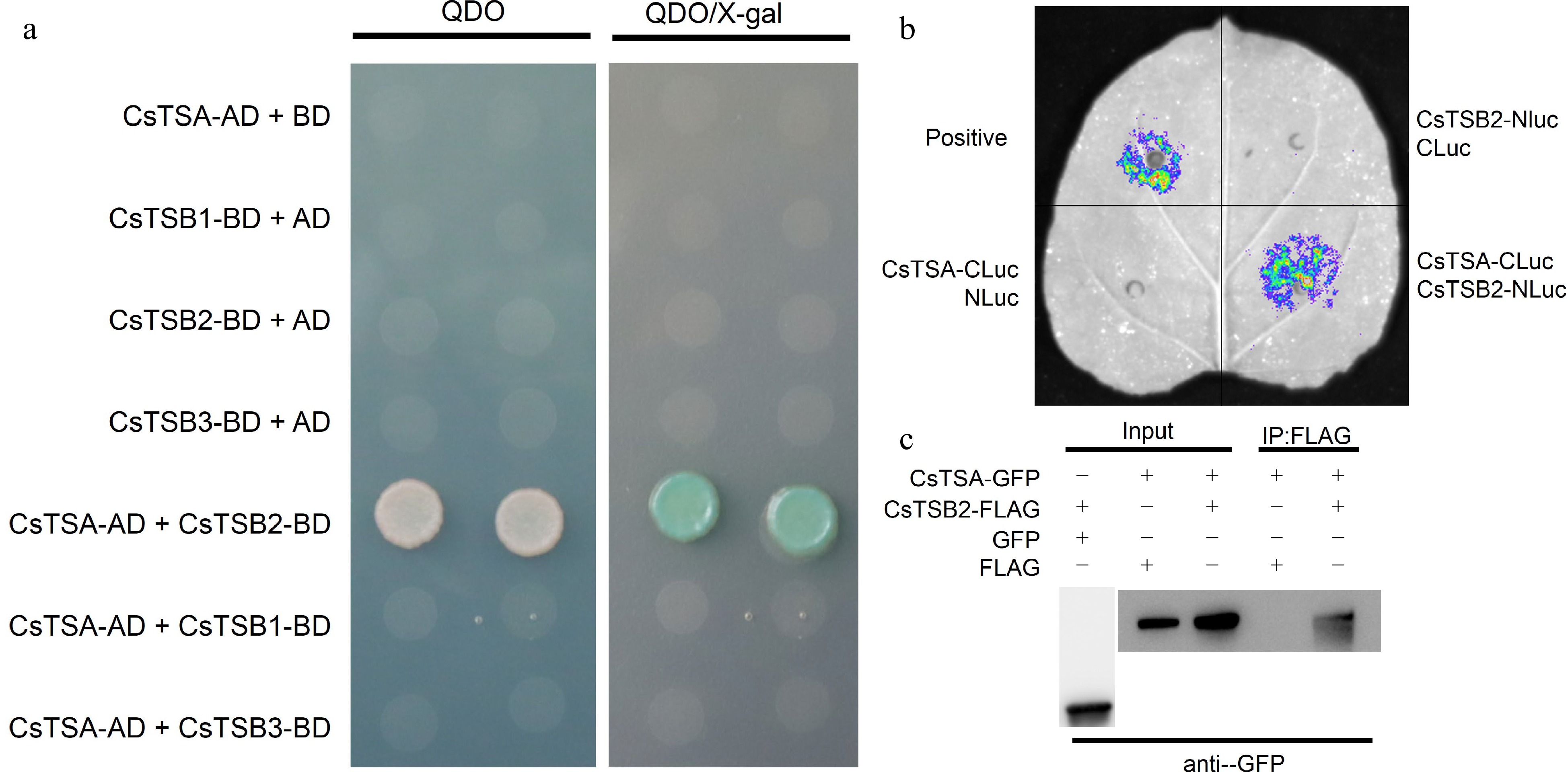
Figure 5.
Interaction between CsTSA and CsTSB2 in vitro and in vivo. (a) Interaction analysis of CsTSA and CsTSB2 in yeast two-hybrid assay. The full-length ORFs of CsTSA and CsTSB2 were fused with AD and BD vectors, co-transfected into yeast cells, and detected for possible interactions in QDO selection medium. QDO, SD/-Leu/-Trp/-His/-Ade. x-gal, X-a-gal. (b) Interaction analysis of CsTSA and CsTSB2 in LCI assay. CsTSA-CLuc and CsTSB2-NLuc were cotransformed into tobacco leaves, and CsTSA-CLuc and CsTSB2-NLuc were cotransformed with NLuc or CLuc, respectively, as a negative control, and fluorescence signals were detected by spraying the lower epidermis of the leaves with fluorescein (100 μM). (c) Interaction analysis of CsTSA and CsTSB2 in Co-IP assay. Total proteins were extracted from tobacco leaves of co-transformed tobacco, respectively. The protein extracts were incubated with anti-FLAG antibody and then immunoprecipitated proteins were detected by western blotting using anti-GFP antibody. Control proteins were detected with anti-GFP and anti-FLAG antibodies, respectively.
Many genes encode proteins that facilitate the transport of certain substances and regulate cellular functions in processes that involve various interactions (e.g., interactions among transcription factors, RNA, and proteins) as well as chromatin structural changes. These interactions result in a complex regulatory system. Moreover, protein interactions allow for complex physiological activities[40]. An earlier analysis of the indole biosynthesis pathway in plants suggested that TSA and TSB form a catalytically active tetramer[41]. Four candidate genes potentially involved in indole synthesis were identified in tea plants, among which CsTSB2 is a key gene for the response to damage. In addition, indole production involving CsTSB2 depends on CsTSA. Subcellular localization experiments confirmed that both CsTSA and CsTSB2 are localized in chloroplasts[4], suggestive of a possible interaction between CsTSA and CsTSB2. In this study, yeast two-hybrid, LCI, and Co-IP assays were performed to examine intracellular protein interactions[42]. Because the available methods for analyzing protein interactions have certain advantages and disadvantages, using multiple methods may ensure protein–protein interactions are accurately detected[43]. In this study, the interaction between CsTSA and CsTSB2 was verified in vitro and in vivo by combining the results of yeast two-hybrid, LCI, and Co-IP assays (Fig. 5), providing the basis for the precise functional characterization of these two proteins in future studies.
-
We analyzed the anti-insect effects of the common HIPV indole as well as the regulated emission of indole by tea plants attacked by tea geometrids. The results of our analyses of indole were consistent with the related findings of a previous investigation[11]. Specifically, indole is a signaling molecule that induces defense-related responses after an infestation by herbivores. Furthermore, it can attract the natural enemies of insect pests (Fig. 6). The emission of indole gradually increased in daytime and decreased at night in response to a simulated herbivore attack that eliminated the effects of herbivore behavior. Indole release was also significantly greater during the day than at night. The regular emission of indole might be associated with its regular synthesis. The endogenous indole content and the expression of CsTSB2 in tea leaves were essentially consistent with the emission of indole. Because of the cooperative relationship between CsTSB2 and CsTSA, the regular expression of CsTSB2 throughout the day may be associated with the observed indole synthesis and emission patterns (Fig. 6). The results of this study have further elucidated how HIPVs, including indole, protect tea plants from insect pests, while also providing useful insights into indole biosynthesis in plants.
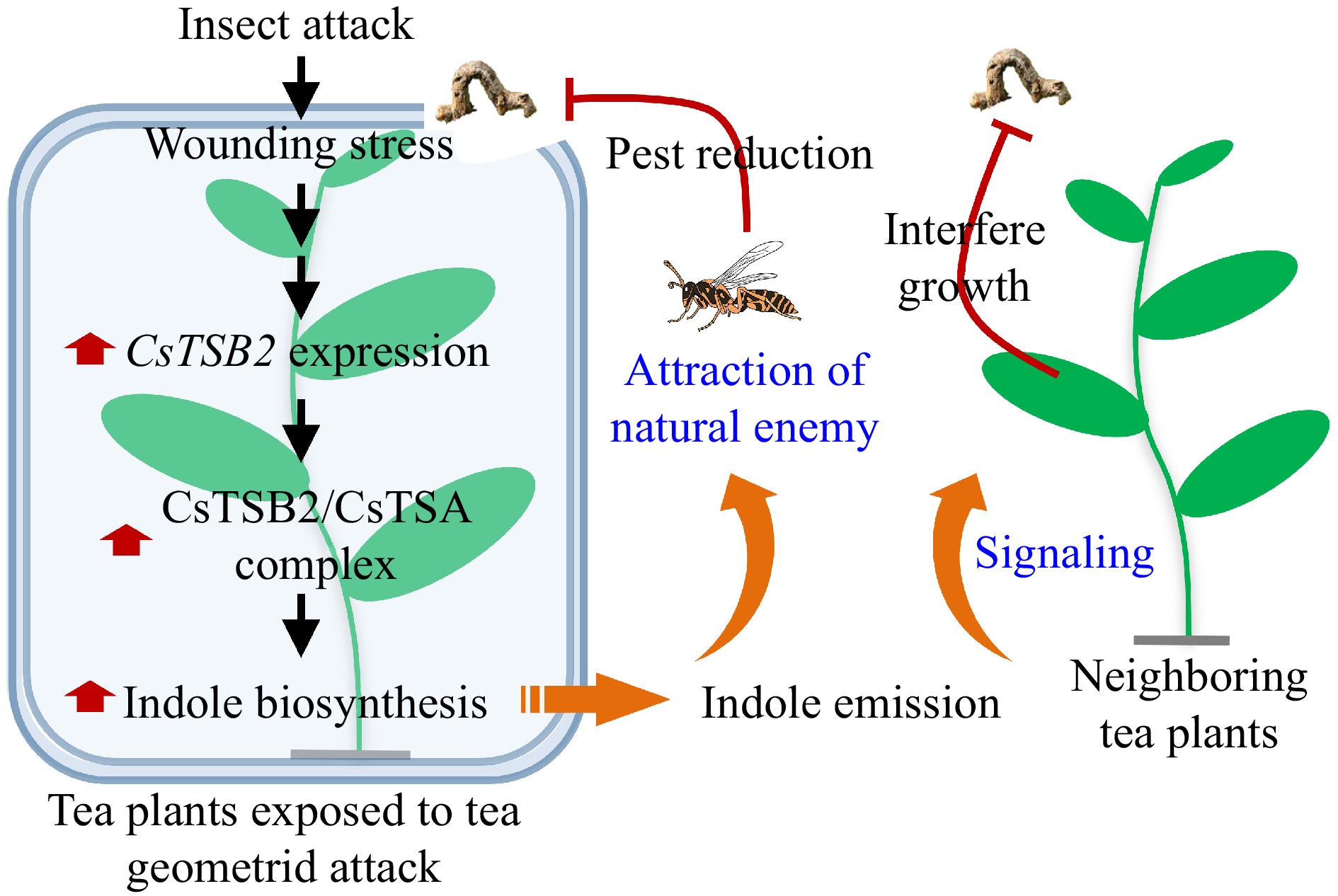
Figure 6.
Insect attack induces emission of indole with indirect anti-insect function in tea plant. Insect attack significantly up-regulated the expression of CsTSB2, a key synthesis gene of indole. CsTSB2 formed a complex with CsTSA to co-regulate indole synthesis. When released, indole improved the ability of neighbouring plants to resist pests by signalling. Indole also acted as an attractant to attract natural enemy of the pest. CsTSA, tryptophan synthase α-subunit; CsTSB, tryptophan synthase β-subunit.
-
The authors confirm contribution to the paper as follows: study conception and design: Zeng L, Yang J; data collection: Li J, Jian G, Yang J; analysis and interpretation of results: Li J, Jian G, Yang J; draft manuscript preparation: Li J, Jian G, Qian J, Xue J, Liu C, Jia Y, Zhou B, Tang J, Yang J, Zeng L. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are not publicly available due to management requests, but are available from the corresponding author on reasonable request.
This study was supported by the financial support from the financial supports from the Guangdong Basic and Applied Basic Research Foundation (2021A1515010930, 2023B1515020107 and 2022A1515011216), the fund for China Agriculture Research System (CARS-19), Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (R2023PY-JG022), the Science and Technology Project of Guangzhou (202206010185), the Guangdong Forestry Bureau (Key Laboratory of ex situ plant protection and utilization in South China) (E336030011), the Foundation of Science and Technology Program of Guangzhou (Major Research Project in South China National Botanical Garden, E3330900), and the Guangdong Provincial Key Laboratory of Applied Botany (South China Botanical Garden) (2023B1212060046).
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Jianlong Li, Guotai Jian
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li J, Jian G, Qian J, Xue J, Liu C, et al. 2024. Emission pattern and anti-insect function of indole from tea plant (Camellia sinensis) attacked by tea geometrids. Beverage Plant Research 4: e003 doi: 10.48130/bpr-0023-0036
Emission pattern and anti-insect function of indole from tea plant (Camellia sinensis) attacked by tea geometrids
- Received: 07 September 2023
- Revised: 26 October 2023
- Accepted: 31 October 2023
- Published online: 02 January 2024
Abstract: Tea plant (Camellia sinensis) are used to produce beverages that are consumed worldwide. Similar to many other plants, tea plant attacked by herbivores emit herbivore-induced plant volatiles (HIPVs) that enhance defense responses, regulate insect behavior, and send 'warning signals' to neighboring plants. However, the related mechanisms remain relatively unclear. In this study, the anti-insect effects and emission patterns of indole, which is a common HIPV synthesized in tea plant, were investigated. The anti-insect effects of indole may involve the induction of signal transduction pathways and the attraction of natural enemies. An attack by tea geometrids, which are major tea plant pests, resulted in the regular emission of indole. The analysis of plants with simulated insect feeding-induced damage showed that indole emission was higher at noon than at midnight. Additionally, significantly more indole was released by plants maintained under continuous light than by plants kept in darkness. The regular emission of indole may be related to the regular expression of the structural gene CsTSB2. The interaction between CsTSA and CsTSB2 was confirmed, further indicating CsTSB2 likely regulates the regular biosynthesis and emission of indole. The study findings may be relevant to improving environmentally friendly pest control measures in tea plantations.
-
Key words:
- Camellia sinensis /
- Indole /
- Anti-insect function /
- Regular emission /
- Protein interaction













