-
Starch is the most important ingredient of human nutrition and also a critical raw material for various industries[1]. During its production, the separation of starch is the most critical step as it determines its purity and quality. The two methods used most in the starch separation process are natural sedimentation and centrifugal separation. The natural sedimentation method has the advantages of lower production costs, lower energy consumption, and better end-product quality, but the process is often time-consuming[2]. Therefore, in recent years, starch flocculant agents, both chemical and microbial flocculants, have been explored to accelerate starch sedimentation and improve production efficiency[3].
Microbial flocculant (MBF) is a class of microorganisms or their metabolites displaying flocculating activity. It provides several advantages over chemical flocculants, such as being widely available, safe, easy to decompose, and free from secondary pollution[4]. Many microorganisms and their metabolites have been studied and utilized as MBF, but their flocculation mechanisms are not yet clearly explained. Four primary mechanisms have been proposed: charge neutralization, adsorption and bridging, chemical reaction, and volume sweeping[5]. In the charge neutralization mechanism, positively charged MBF can attract negatively charged colloids in solution by electrostatic forces. The neutralizing effect reduced the zeta potential and repulsive forces between the colloid particles, forming large flocs by van der Waals, followed by sedimentation due to gravity. The adsorption bridging mechanism often refers to establishing a bridge-like three-dimensional structure between colloids and MBF by hydrogen bonding and ionic bonding through MBF's carboxyl, hydroxyl, amino groups, et al. In contrast, the chemical reaction mechanism proposes that the MBF molecule contains some reactive groups that can react chemically with the colloidal particles in the medium to form larger flocs. Volume sweeping mechanism can be used to explain the continuous increase of the flocculation floc during sedimentation, in which flocs can sweep and capture the particles around them to form a large floc. Flocculation is a complicated process, and these mechanisms may take place at the same time. MBF is intensively researched and widely applied in many fields. One of the new trends for the application of MBF is in food production, as researchers have utilized MBF in clarifying sugar liquor and separating fermented products[6].
Using MBF for starch production is green, energy-saving, and efficient. So far, the starch MBFs are mainly lactic acid bacteria (LAB) derived from naturally fermented starch emulsions, such as Streptococcus lactis[7], Leuconostoc mesenteroides[8], Acetobacter indonesiensis[9], and Lactobacillus paracasei subsp. paracasei L1[3]. Our previous research reported for the first time that a Leuconostoc citreum strain (SJ-57) could also serve as an efficient MBF in starch production. Using this strain could shorten the traditional production time of sweet potato starch from 6-8 h to 3 h with high purity[10]. All these researches have demonstrated the great potential of MBF to improve the efficiency of starch production. However, the mechanism of starch flocculation by MBF is yet to be unveiled.
Zhang et al.[3] suggested that the starch-flocculation properties of MBF could be the result of the bacterial cells' starch-binding ability. The bacterial starch-adhesive ability has been investigated in Vibrio cholerae, Bacteroides thetaiotaomicron, and other lactic acid bacteria, revealing that the starch-bind ability might be associated with bacterial surface proteins[11−15]. For example, it was reported by Shipman et al.[12] that outer membrane proteins, namely SusC, SusD, SusE, and SusF, were essential to starch-adhesive properties of Bacteroides thetaiotaomicron. However, these studies failed to specify the interactions between the cells and the starch granules with only partial evidence. It was found that the interaction of Bifidobacterium spp. with starch was independent of electrostatic interaction[11]. Furthermore, Lactobacillus amylovorus's ability to utilize starch may serve as the fundamental to its ability to bind with starch[13] . As starch granules adhere to Vibrio cholerae cells, non-specific hydrophobic interactions arose on their surfaces, along with some specific carbohydrate adhesion molecules[14]. The mechanism behind starch flocculation can only be better understood if the interactions between the particles and bacteria are further elucidated.
Recently, we have identified and reported for the first time that the starch-flocculating activity of Leuc. citreum SJ-57 originated from their cell surface components[10]. Critical questions remain unanswered: which cell surface components are the key contributor to its flocculating activity, and what interactions caused the flocculation? In this study, Fourier-infrared spectroscopy, zeta potential, binding inhibitors, and thermodynamic theory were introduced to systematically explore the short-range and long-range interactions between bacteria and starch. Thermal, enzymatic, and chemical treatments were used to isolate the effect of various cell surface components on the flocculation. Surface proteins involved in the cell-starch binding were also identified by mass spectrometry and SDS-PAGE. These results complement the theory of the flocculation mechanism of MBF and provide a foundation for the utilization of Leuc. citreum as a potential novel MBF.
-
Leuc. citreum SJ-57 (CGMCC NO. 19201) was isolated from sweet potato sour liquid, as reported previously[10]. Strains of bacteria were streaked on MRS agar plates (Aobox, China) and incubated at 37 °C for 16 h. MRS broth was inoculated with a colony from the agar plate to grow pre-cultures under the same conditions. A second culture was inoculated with pre-cultures and grown for 16 h before harvesting. The solution was centrifuged at 8,000 g for 10 min, rinsed twice with deionized (DI) water, and then suspended in sterile saline (~109 CFU/mL) for future experiments.
Determination of flocculating rate (FR)
-
The FR of the Leuc. citreum SJ-57 was determined by measuring turbidities of the starch emulsion according to our previous method[10]. Add 2 mL of testing culture to 25 mL of sweet potato starch solution (25 g/L) and then take an aliquot of 0.1 mL to measure the OD500 after 20 min of reaction at room temperature. The FR was determined with sterile saline solution undergoing the same procedure as the control:
$ FR\left({\text%}\right)=\frac{{OD}_{550\;control}-{OD}_{550\;test}}{{OD}_{550\;control}}\times 100{\text%} $ Effect of thermal and enzymatic treatments on the flocculating activity of Leuc. citreum SJ-57
-
Cells (~109 CFU/mL) were heated in the water bath at 25, 30, 35, 40, 45, 55, and 60 °C for 30 min. Cells were treated with 1 mg/mL of the following three enzymes at 37 °C for 30 min when pH = 7, respectively. 1) proteinase K from Tritirachium album, 2) β-dextranase from Chaetomium erraticum, and 3) lipase from Aspergillus. Cells were also treated with 400 μg/mL lysozyme for 15 min at 37 °C when pH = 6. After enzymatic treatments, the bacteria were rinsed three times with DI water and suspended in sterile saline (~109 CFU/mL). The untreated cells were used as the control group.
Removal of surface proteins and exopolysaccharides (EPS)
-
Washed cells were incubated with 5 M guanidine hydrochloride (GHCl) in a shaking incubator (150 rpm) for 30 min at 37 °C to remove surface protein before being rinsed with sterile saline[16]. The cells of the control group were untreated.
After washing, cells were treated with 1 M NaCl in a shaking incubator (150 rpm) for 0, 5, 10, 20, 40, 60, and 120 min at 37 °C before being centrifuged for 15 min at ×8,000 g. The centrifuged cells were resuspended in sterile saline. The supernatants of each treatment were lyophilized for the detection of the contents of carbohydrates in total and protein in supernatants by a phenol sulfuric acid method[17] and the Bradford method[18].
Isolation of starch-binding proteins
-
Surface proteins were extracted from bacterial cells according to a method by Malamud et al.[16] with modification. Briefly, membranes with 0.45 μm pores were used to filter the supernatant after the bacteria were centrifuged (15 min at ×8,000 g) with 5 M GHCl. The protein-containing solutions were dialyzed at 4 °C using distilled water and then stored at −80 °C after freeze-drying. Sweet potato starch (10 mg) was added to 1 mL of surface proteins in PBS (1 mg/mL). The mixture was stirred for 30 min to allow full exposure before unbound proteins were removed by centrifugation (15 min at ×8,000 g). The Bradford method was applied to measure the protein concentration of the supernatant. Then, 40 μL of loading buffer was added to the centrifuged starch granules in 60 μL of PBS. In order to remove starch-binding proteins from the starch granules, samples were heated in boiling water for 10 min[19]. The boiling solution was used for 10 min to resuspend whole surface proteins, unbound surface proteins, and starch-binding surface proteins for SDS-PAGE. Each sample was then run through 20 μL. After digestion with trypsin, the samples were examed with liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) by Q Exactive (Thermo Scientific, USA) and analyzed by a MaxQuant (1.6.2.10) protein identification program.
Transmission electron microscopy (TEM)
-
Fixation of cells in 0.05 M phosphate buffer (pH 7.2) with 2.5% glutaraldehyde was conducted. After washing with the phosphate buffer, the samples were fixed overnight at 4 °C with 1% osmium tetroxide in Kellenberger and Ryter's veronal-acetate buffer, followed by 0.5% uranyl acetate for 2 h. Epoxy resin was embedded in the specimens after dehydration through acetone. The samples were sliced by a Sorval Porter-Blum MT-1 ultramicrotome, stained with lead citrate, and observed in an 85-kV JEOL 12000EXII TEM (Jeol Ltd.)[20].
Examination of the interactions between cells and starch granules
-
To investigate the hydrogen bond and ionic bonds between starch and bateria, the washed bacterial cells were suspended in KCl (10 mmmol/L) and CaCl2 (10 mmmol/L), respectively, and then attached to starch granules by mixing with 25 g/L starch granules as described above. The supernatant was discarded after complete sedimentation for 20 min, and the starch sediment was pretreated with 2 mL of 5 M urea, 10 mmol/L HCl, and 10 mmol/L EDTA for 5 min at 20 °C, respectively, before being reconstituted into a starch emulsion (25 g/L, pH = 7) by adding KCl (10 mmmol/L) and CaCl2 (10 mmmol/L), respectively. The FR of each treatment was determined as above.
Zeta potentials in various flocculation systems
-
The Zeta potential of the bacterial cells (~108 CFU/mL) and starch granules (1 g/L) were measured in the presence of 0–10 mM of KCl, NaCl, CaCl2, MgCl2 at pH = 7 using a Malvern Zetasizer Nano System (Brookhaven Instruments Corporation, USA)[21].
Fourier Transform-Infrared (FT-IR) spectroscopy
-
The chemical compositions of Leuc. citreum SJ-57 surface, starch granules, and bacteraia-adhered starch granules were examined using a Bio-Rad FTS 135 spectrometer (Hercules, CA, USA) in the reflectance mode, ranging 400 to 4,000 cm−1.
Contact angle measurement and surface energy estimation
-
The contact angles of bacteria and starch were analyzed using the sessile drop method. Briefly, rinsed cells were kept in 10 mM KCl at pH = 7 for 4 h and then harvested by filtration through cellulose acetate membranes with 0.45 mm pore sizes (Beijing Solarbio Science & Technology Co., Ltd., China) to create a cell layer and air-dried at room temperature. Starch granules were also kept in 10 mM KCl at pH = 7 for 4 h and then lyophilized for detection. The probe liquids used and their properties are summarized in Table 1.
Table 1. Surface tension parameters of probe liquids used for contact angle measurements for Leuc. citreum SJ-57 and starch granules.
Surface tension parameters (mJ/m2) γLW γ+ γ− γTot H2O 21.8 25.5 25.5 72.8 C3H8O3 34.0 3.9 57.4 64.0 CH2I2 50.8 0.0 0.0 50.8 Note: γLW represents the LW component of the surface tension. γ+ and γ- are the electron-acceptor and electron-donor parameters of the surface tension, respectively. The hydrophobicity of bacteria and starch was expressed in ΔGiwi, with i representing particles and w representing water[22].
$ {\Delta G}_{iwi}={-2\left(\sqrt{{\mathrm{\gamma }}_{i}^{LW}}-\sqrt{{\mathrm{\gamma }}_{w}^{Lw}}\right)}^{2}-4\left(\sqrt{{\mathrm{\gamma }}_{i}^+}-\sqrt{{\mathrm{\gamma }}_{w}^-}\right)\left(\sqrt{{\mathrm{\gamma }}_{i}^+}-\sqrt{{\mathrm{\gamma }}_{w}^-}\right) $ (1) Where γLW is the Lifshitz–van der Waals (LW) parameter of the surface tension (mJ/m2), and γ+ and γ− are the electron-acceptor and electron-donor parameter (mJ/m2), respectively.
Contact angles were measured with three different probe liquids (one non-polar and two polar), and Young's Equation was applied to determine the three unknown entities
$\gamma_i^{LW} $ $\gamma_i^+ $ $\gamma_i^- $ $ {\mathrm{\gamma }}_{l}^+(1+\mathrm{cos}{\theta }_{li})=2\left(\sqrt{{\mathrm{\gamma }}_{l}^{Lw}{\mathrm{\gamma }}_{i}^{LW}}+\sqrt{{\mathrm{\gamma }}_{l}^+{\mathrm{\gamma }}_{i}^-}+\sqrt{{\mathrm{\gamma }}_{l}^-{\mathrm{\gamma }}_{i}^+}\right) $ (2) where θ is the contact angle, and the indices l and i denote solids and liquids.
Interaction energy calculations
Calculation of Leuc. citreum SJ-57–starch interaction energy profiles
-
The Derjaguin, Landau, Verwey, and Overbeek (DLVO) theory was applied to calculate the interaction energies between the bacteria and starch[22]. By considering the system using the sphere-slab model, total interaction energies were calculated as the sum of three interactions[24].
$ {G}_{}^{Tot}\left(d\right)={G}_{}^{LW}\left(d\right)+{G}_{}^{EL}\left(d\right)+{G}_{}^{AB}\left(d\right) $ (3) Where LW, EL, and AB denote van der Waals, electrostatic interactions, and Lewis acid/base interactions, respectively.
GLW(d) was calculated using the following expression:
$ {G}_{1W2}^{LW}\left(d\right)=\frac{-{A}_{1W2}{r}_{1}}{6d}\left[1+\frac{d}{2{r}_{1}+d}+\frac{d}{r}\mathrm{l}\mathrm{n}\left(\frac{d}{2{r}_{1}+d}\right)\right] $ (4) Where r1 is the equivalent bacterial radius (m). A1w2 is the Hamaker constant (J), a combination of bacteria (1) and starch (2) in water (w), calculated via Eqn (5):
$ {A}_{1w2}=-24\pi {d}_{0}^{2}\left(\sqrt{{\gamma }_{1}^{LW}}-\sqrt{{\gamma }_{W}^{LW}}\right)\left(\sqrt{{\gamma }_{2}^{LW}}-\sqrt{{\gamma }_{W}^{LW}}\right) $ (5) GEL(d) was formulated as[23]:
$ \begin{aligned}{G}_{1w2}^{EL}\left(d\right)=&\;\pi \epsilon {r}_{1}\Bigg\{2{\psi }_{1}{\psi }_{1}ln\left[\frac{1+\mathrm{e}\mathrm{x}\mathrm{p}(-\kappa d)}{1-\mathrm{e}\mathrm{x}\mathrm{p}(-\kappa d)}\right]\\&+\;\left({\psi }_{1}^{2}+{\psi }_{1}^{2}\right)ln\left[1-\mathrm{e}\mathrm{x}\mathrm{p}(-2\kappa d)\right]\Bigg\} \end{aligned}$ (6) Where ε is the dielectric constant of water; ψ1 and ψ2 are the surface potential of the starch and bacterium, respectively, which are assessed from zeta potentials based on ψ = zeζ/κT; z represents the ion valence.
As shown in Van Oss[23], κ is the reverse of the Debye length (m−1):
$ \mathrm{\kappa }=\sqrt{\frac{2{e}^{2}{N}_{A}I}{\mathrm{\epsilon }{K}_{B}T}} $ (7) Where e represents the elementary charge; NA denotes Avogadro's number; kB is Boltzmann's constant; T is the absolute temperature; I denotes the solution ionic strength.
This equation was used to calculate GAB(d):
$ {G}_{1W2}^{AB}\left(d\right)=2\pi {r}_{1}\lambda \Delta {G}_{d0}^{AB}\mathrm{e}\mathrm{x}\mathrm{p}\left(\frac{{d}_{0}-d}{\lambda }\right) $ (8) Where λ is the decay length of AB interactions. d0 is the minimum equilibrium distance. From the surface tension parameters, ΔGAB d0 can be determined as the Lewis acid/base free energy of the bacterium-starch interaction at a minimum separation distance (d0).
$\begin{aligned} {\Delta G}_{d0}^{AB}=\;&\;2\left(\sqrt{{\gamma }_{1}^+}-\sqrt{{\gamma }_{2}^+}\right)\left(\sqrt{{\gamma }_{2}^-}-\sqrt{{\gamma }_{2}^-}\right)\\&-\;2\left(\sqrt{{\gamma }_{1}^+}-\sqrt{{\gamma }_{w}^+}\right)\left(\sqrt{{\gamma }_{1}^-}-\sqrt{{\gamma }_{w}^-}\right) \\&-\;2\left(\sqrt{{\gamma }_{2}^+}-\sqrt{{\gamma }_{w}^+}\right)\left(\sqrt{{\gamma }_{2}^-}-\sqrt{{\gamma }_{w}^-}\right)\end{aligned} $ (9) Calculation of bacteria-bacteria and starch-starch interaction energy profiles
-
The DLVO theory was used to obtain the interaction energy between cell-cell and starch-starch. The interaction energies were calculated with the sphere–sphere model as follows[23]:
$ {G}_{1w2}^{LW}\left(d\right)=\frac{-{A}_{1w2}{r}_{1}{r}_{2}}{6d({r}_{1}+{r}_{2})} $ (10) $\begin{aligned} {G}_{1w2}^{EL}=\;&\;\pi \epsilon \frac{{r}_{1}{r}_{2}({\psi }_{1}^{2}+{\psi }_{2}^{2})}{({r}_{1}+{r}_{2})}\Bigg\{\frac{2{\psi }_{1}{\psi }_{2}}{{\psi }_{1}^{2}+{\psi }_{2}^{2}}ln\left[\frac{1+\mathrm{e}\mathrm{x}\mathrm{p}(-\kappa d)}{1-\mathrm{e}\mathrm{x}\mathrm{p}(-\kappa d)}\right]\\&+\;ln\left[1-\mathrm{e}\mathrm{x}\mathrm{p}(-2\kappa d)\right]\Bigg\} \end{aligned}$ (11) $ {G}_{1w2}^{AB}\left(d\right)=\pi \lambda \frac{{r}_{1}{r}_{2}}{({r}_{1}+{r}_{2})}\Delta {G}_{d0}^{AB}\mathrm{e}\mathrm{x}\mathrm{p}\left(\frac{{d}_{0}-d}{\lambda }\right) $ (12) Where r1 and r2 are the equivalent bacterial radius (m) and starch radius (m), respectively, and the remaining parameters were the same as above.
Statistical analysis
-
All experiments were conducted in triplicate, and the results were reported as mean ± standard deviation. One-way analysis of variance with Duncan's test was performed with IBM SPSS Statistics 25 (SPSS Inc., Chicago, IL, USA) with α = 0.05.
-
As the size of bacteria (0.5−2 μm) is within the category of colloidal particles (0.5−500 μm), both short-range interactions (< 1 nm) and long-range interactions (1−100 nm) should be considered to study the interactions between bacteria and starch granules[22]. The long-range interaction determines whether the two interfaces or particles are sufficiently close to each other for short-range interactions to occur. The short-range interaction determines whether the particles are reversibly or irreversibly bound together[25].
FT-IR was used for the preliminary qualitative study with the short-range interactions between Leuc. citreum SJ-57 and starch granules. Figure 1a showed the representative peaks of starch granules before and after they adhered to Leuc. citreum SJ-57. A similar pattern between them suggested that the starch granules did not form new chemical bonds before and after the flocculation. Thus, bacteria did not interact with the starch via covalent bonds. The -OH vibrational absorption peak of the bacteria-starch complex was blue-shifted from 3,507 cm−1 to 3,463 cm−1 compared to that in the pure starch granules (Fig. 1a), implying that hydrogen bonds might be formed between starch granules and Leuc. citreum SJ-57. In addition, the absorption peak of the Amide I band of the bacteria-starch complex was shifted from 1,651 cm−1 to 1,653 cm−1, inferring the existence of electrostatic interaction between the starch and bacteria cells. Dai et al.[26] reported a similar Amide I band shift when zein was bound to soy lecithin due to electrostatic interaction.

Figure 1.
FT-IR spectra of (a) untreated starch & Leuc. citreum SJ-57 adhered starch and (b) untreated Leuc. citreum SJ-57 & starch-adhered Leuc. citreum SJ-57. (c) The effect of different metal ion concentrations and types on Leuc. citreum SJ-57's FR, (d) the zeta potential of starch, and (e) the zeta potential of bacteria. (f) Effects of chemical treatments on Leuc. citreum SJ-57's FR: The floc were formed in 0.01 mol/L KCl (I), 0.01 mol/L KCl and 0.01 mol/L HCl (II), 0.01 mol/L KCl and 5 mol/L urea (III), 0.01 mol/L CaCl2 (IV), and 0.01 mol/L CaCl2 and 0.01 mol/L EDTA (V). Statistical analysis of differences between FRs with cells after various treatments performed using t-test. * represents p < 0.05.
In order to examine the changes in the bacterial surface groups, we obtained the spectra of the bacteria using the spectral difference subtraction technique (Fig. 1b). The characteristic peaks showed that the surface of Leuc. citreum SJ-57 cell was dominated by polysaccharides, lipids, and proteins, which was consistent with the known composition of Leuc. citreum[27]. The absorption spectra showed no new characteristic peaks when comparing starch-bound bacteria with pure bacteria (Fig. 1b), suggesting that Leuc. citreum SJ-57 was attached to starch granules via only non-covalent bonds. Noteworthily, only the bacterial peaks corresponding to amide I, amide II, and amide III bands were differentially displaced (> 4 cm−1) after the bacteria was bound to the starch granules, implying an alteration in bacterial proteins' secondary structure during starch flocculation and the involvement of electrostatic interactions between Leuc. citreum SJ-57 and the starch granules[28]. Hence, we speculated that the short-range interactions between Leuc. citreum SJ-57 and starch granules were mainly hydrogen bonding and electrostatic interactions.
FR and zeta potential of cells and starch granules in different ionic strength
-
We started to investigate the potential electrostatic interactions by observing how metal cations impacted the starch-flocculating activity of Leuc. citreum SJ-57. The strain could not flocculate any starch granules in pure DI water with no ions, whereas FR started to rise with increasing metal ion concentration (Fig. 1c), suggesting Leuc. citreum SJ-57 required metal ions to flocculate starch. It was also evident that divalent metal cations (Ca2+ and Mg2+) led to higher FR than monovalent ones (K+ and Na+), suggesting that metal cations might have an electrical neutralization effect. Noteworthily, at the same concentration, KCl incited higher flocculation activity of Leuc. citreum SJ-57 starch than NaCl So is CaCl2 when compared with MgCl2. It might be related to the fact that metal cations with a larger radius (K+ and Ca+) had a greater ability to compress the double layer and form interparticle aggregation[29].
The zeta potentials of cells and starch granules in different solutions were used to explore the effect of metal cations, as shown in Fig. 1d and e. The surface potentials of starch granules and Leuc. citreum SJ-57 were both below -30 mv in the DI water, revealing a considerable electrostatic repulsion between them. According to the theory of colloid chemistry, these particles are stable and hard to aggregate[30]. When metal cations were introduced, the surface potential of starch granules and bacterial cells increased with the higher concentration of metal cations (Fig. 1d & e), possibly because metal cations can partially neutralize the negative charge on the surface of the cells[24]. At the same concentration, K+ and Ca2+ can neutralize slightly more charge on the bacteria and starch than Na+ and Mg2+, respectively, suggesting they can compress the double electric layer more effectively due to greater ionic radii[29]. In conjunction with the results above, we speculated that the primary role of the metal cations in the matrix was to form ionic bonds between Leuc. citreum SJ-57 and starch.
Disruption of the interactions between cells and starch
-
In order to reveal the possible hydrogen bonds and metallic ion bonds between Leuc. citreum SJ-57 and starch, we treated the pre-formed starch-bacteria flocs with ionic bonds blocker (HCl and EDTA) and hydrogen bond blocker (urea) to test whether the flocs would disaggregate. The results are presented in Fig. 1f. It is evident that adding HCl and EDTA disrupted the starch-bacteria flocs, resulting in significant decreases in flocculation activity. It also showed that the flocs were dispersed in the first group after adding an equal amount of HCl to the flocs as NaCl in the system. Similarly, the flocs of the second group were found to be dispersed after adding an equal amount of EDTA to the CaCl2 in the system. These results suggested that metal ionic bonds were formed between Leuc. citreum SJ-57 and the starch granules. According to the FT-IR analysis, there was a large amount of -OH and -COOH on the surface of the starch granules. The characteristic peaks of both groups produced shifts representing electrostatic interactions after flocculation (Fig. 1f). In addition, the characteristic peaks of the amide band on the bacterial surface were shifted to varying degrees upon adherence to starch, especially at 1,654 cm−1, where the characteristic peak representing -COO- was easily the coordination bond with the metal cation[30]. Thus, we hypothesized that metal cations linked cells and starch granules by simultaneously connecting the -O- of the starch granules to the -COO- of the surface proteins of Leuc. citreum SJ-57. The effect of pH on the FR of Leuc. citreum SJ-57 is illustrated in Supplemental Fig. S1. The pH-induced change of FR further validate our hypothesis.
Urea contains C=O, which can form hydrogen bonds with hydrogen. If there are hydrogen bonds between the particles, the hydrogen bonds will break, and new hydrogen bonds will be made with urea[29]. Adding 5 mol/L of urea to the floc was not able to disrupt it (Fig. 1f). This result indicated that no hydrogen bonds were formed between Leuc. citreum SJ-57 and the starch granules.
These findings collectively demonstrated that hydrogen bonding between Leuc. citreum SJ-57 and starch granules were not particularly evident. Similar findings were reported that the flocculation of starch by L. paracasei subsp. paracasei L1 was mainly through ionic bonding[3]. The ionic bond is the strongest non-covalent bond, with a bond energy closer to that of the covalent bonds[25]. Therefore, the formation of starch-Leuc. citreum SJ-57 floc was strong and irreversible, which was difficult to interrupt.
Long-range interaction between cells and starch granules
-
When analyzing the long-range interactions between microorganisms and solid particles, DLVO theory is used to conduct the calculation[22]. Table 2 presents the liquid-solid contact angle of Leuc. citreum SJ-57 and starch granules in different solutions and their calculated hydrophobicities. The water contact angle of Leuc. citreum SJ-57 was much larger than that of the starch granules, indicating higher hydrophobicity of the bacterial cells than starch[23]. ΔGiwi often represents the hydrophobicity of the colloidal particles. If ΔGiw > 0, the colloidal particles are hydrophilic, and ΔGiw < 0 indicates hydrophobic colloids[31]. The ΔGiwi of Leuc. citreum SJ-57 was −15.8 mJ/m2, representing a hydrophobic surface. The starch granule has ΔGiwi of 13.7 mJ/m2, a hydrophilic surface. It was consistent with the common knowledge that most bacteria had hydrophobic surfaces and that starch was a hydrophilic component[24, 32].
Table 2. Contact angles of water, glycoside, and diiodomethane on Leuc. citreum SJ-57 and starch granules, along with the derived surface tension parameters and hydrophobicity of the surfaces.
Contact angles (degree) Surface tension
parameters (mJ/m2)ΔGiwi
(mJ/m2)H2O C3H8O3 CH2I2 γLW γ+ γ− Leuc. citreum
SJ-5753.5 ± 4.3 49.2 ± 2.0 24.5 ± 2.9 46.3 0.3 21.9 −15.8 Starch 26.8 ± 2.8 31.3 ± 7.5 18.5 ± 2.7 48.2 41.6 0.6 13.7 The contact angles are presented as the means ± standard error. Parameters γLW, γ+, and γ− are the surface tension components. Hydrophobicity was evaluated by ΔGiwi, which was calculated using the surface tension parameters according to Eqn (2). Figure 2a illustrates the change of the total long-range interaction energy (solid line) and the individual partial interaction energy (dashed line) as a function of the distance between Leuc. citreum SJ-57 and starch granules at pH = 7 with 10 mmol/L KCl solution, which is a common condition found in the sedimentation tank of the sweet potato starch plant. The total long-range interaction energies between Leuc. citreum SJ-57 and the starch granules were consistently above 0 when the distance d > 1 nm, and it showed a rapid and substantial increase when d ≈ 1 nm. This suggested an energy barrier between Leuc. citreum SJ-57 and the starch granules, with their long-range interaction as a repulsive force. Such repulsion would prohibit them from spontaneously interacting with each other unless substantial kinetic energy from the outside brought the particles close to each other[25]. Leuc. citreum does not have flagella and, therefore, can not move independently[27]. Due to the large size of the bacteria and starch particles (> 1 μm), they produce very limited Brownian motion in solution[25]. Thus, Leuc. citreum SJ-57 required additional supplementary kinetic energy to cross the energy barrier, i.e., stirring or agitation in the solution, to initiate the starch flocculation.

Figure 2.
Total long-range interaction energy (Tot) and the individual partial interaction profiles between (a) Leuc. citreum SJ-57 and starch, (b) Leuc. citreum SJ-57 and Leuc. citreum SJ-57, (c) starch and starch, in 0.01 mol/L KCl solution at pH = 7 calculated by DLVO theory. FT-IR spectra of (d) untreated Leuc. SJ-57 and (e) starch in 0−10 mmol/L KCl solution. (f) Changes in the equivalent diameter of strains and starch in 0−10 mmol/L KCl solution. LW, EL, and AB denote van der Waals, electrostatic interactions, and Lewis acid/base interactions, respectively.
We then investigated the individual partial interaction energy between the bacteria and starch. The van der Waals interaction was consistently negative, as seen in Fig. 2a, causing an attraction between these two components. In contrast, the electrostatic interaction and hydrophobic interactions were always above 0 and much stronger than the van der Waals interaction, resulting in the overall repulsion in the colloids.
Cell-cell and starch-starch interaction
-
We first observed the IR of Leuc. citreum SJ-57 and starch in different solutions to determine whether they can form strong short-range interactions. It was found that the IR characteristic peaks of the cells and starch did not significantly shift when the surrounding KCl concentration increased from 0 to 20 mmol/L (Fig. 2d & e). Thus, their interactions were mainly regulated by long-range interactions. Figure 2b showed the variation of the total (solid line) and individual partial energies (dashed line) as a function of the distance between bacteria. The total energy between cells behaved as repulsive forces from 1 nm to 100 nm, dominated by strong positive electrostatic interactions since the bacterial cells were negatively charged in the solution (Fig. 1e). And the negative van der Waals and hydrophobic interactions were much weaker. As a result, Leuc. citreum SJ-57 was not prone to aggregate in the KCl solution.
The distance-dependent total and partial interaction energy profiles between starch granules are very similar to those of the bacteria-starch complex (Fig. 2c). The total energy of interaction between the starch granules was repulsive and reached a maximum value at 1 nm. Such a large energy barrier between the starch granules prevented them from forming aggregates. Electrostatic and hydrophobic interactions dominated the total repulsive energy due to its negatively charged surface (Fig. 2c). The aforementioned results were further confirmed by measuring the equivalent diameters of Leuc. citreum SJ-57 and starch granules in KCl solution. Increasing the KCl concentration did not change the diameters of particles (Fig. 2f), suggesting that they did not aggregate. Therefore, we concluded that the starch-flocculating activity of Leuc. citreum SJ-57 directly resulted from bacteria-starch interaction, not self-aggregation.
Starch-flocculating ability of Leuc. citreum SJ-57's cell surface components
The effect of thermal and enzymatic treatments on the starch-flocculating ability of Leuc. citreum SJ-57
-
Our previous study has demonstrated the starch-flocculating activity of Leuc. citreum SJ-57 was associated with its unique cell surface structure[10]. The cell surface of Leuc. citreum, like other LAB, is composed of lipids, proteins, and polysaccharides[27]. The MBFs consisting of polysaccharides are largely unaffected by temperature, whereas the activities of MBFs with proteins as their key component are strongly influenced by temperature since the heat would cause structural alteration of the proteins or even denature them completely[33]. Consequently, in order to understand the contribution of the cell surface components to flocculation, we first examined the thermal stability of the starch flocculation activity of Leuc. citreum SJ-57. As shown in Fig. 3a, the starch flocculation of the bacteria was very heat-sensitive, with the highest FR occurring when the cells were heated to 40°C, followed by a dramatic decrease above 45°C. This result agreed with prior reports on several starch-binding LAB, evidenced by the significant loss of starch flocculation activity in both Lactobacillus paracasei L1 and Streptococcus lactis due to excessive heat[3, 7, 34]. We thus suspected the starch-flocculating ability of Leuc. citreum SJ-57 was associated with its cell surface proteins.
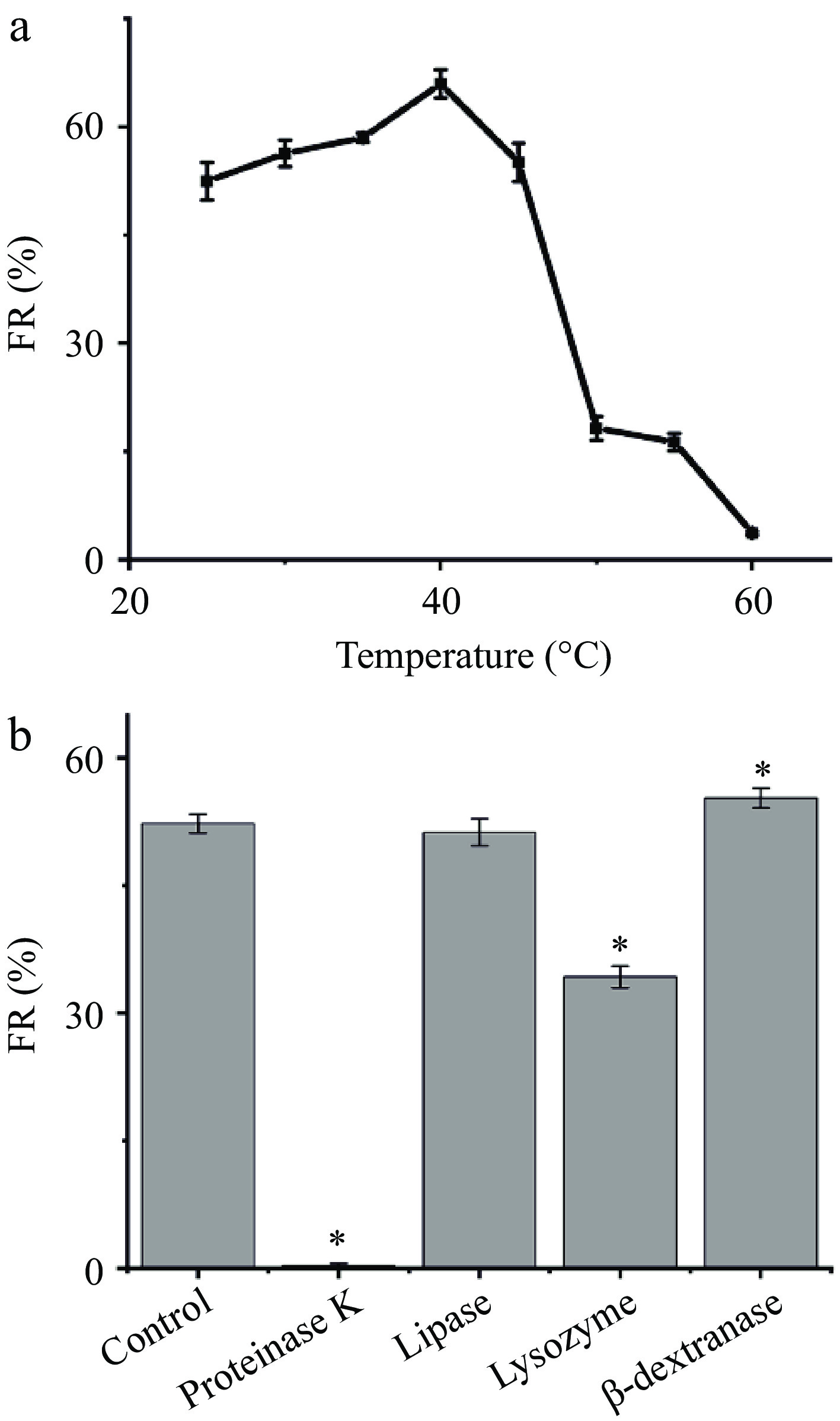
Figure 3.
Effects of (a) thermal treatment and (b) enzymatic treatments of Leuc. citreum SJ-57 on their FR. Statistical analysis of differences in FRs between cells after various treatments and the control group was performed using t-test. * represents p < 0.05.
To further assess the contribution of surface lipids, proteins, and polysaccharides, four different enzymes were used to compromise the cell surface structure: proteases, lipases, β-dextranases, and lysozyme. Figure 3b showed that Leuc. citreum SJ-57 treated with protease K almost lost its flocculating ability entirely, whereas lipase had no effect on the FR of the cells. Proteinase K is a serine protease with broad cleavage activity, which hydrolyzes most proteins[11]. Lipases mainly hydrolyze lipid components into glycerol and fatty acids[3]. Therefore, we inferred that only proteins, not lipids, were associated with starch-flocculation activity, Leuc. citreum SJ-57.
The FR of the lysozyme-treated Leuc. citreum SJ-57 also exhibited a significant decrease, which was inconsistent with the previous report in which the flocculation activity of L. paracasei L1 was not affected by lysozyme[3]. Lysozyme effectively hydrolyses the peptidoglycan in the bacterial cell wall destroying the cell structure[3]. Hence, intact cell structure was crucial to Leuc. citreum SJ-57's ability to flocculate starch. We also found that the FR of the dextranase-treated cells increases slightly. The extracellular polysaccharide (EPS) around Leuc. citreum was mainly dextrose, which can be hydrolyzed by dextranases[35]. This would indicate that the presence of EPS might interfere with Leuc. citreum SJ-57's ability to flocculate starch, thus removing EPS resulted in an increase in FR. Next, we needed to validate further the starch-flocculating mechanism behind each key cell surface component of Leuc. citreum SJ-57.
Cell surface proteins' starch binding capacity
-
The cell surface proteins played a key role in the starch-flocculating ability of Leuc. citreum SJ-57 via binding with the starch granules. Thus, guanidine hydrochloride (GHCl) was used to remove and extract the cell surface proteins without compromising the cell viability, as GHCl can break the non-covalent bond between proteins and peptidoglycan in the cell wall[20]. The total loss of FR due to GHCl in Fig. 4a confirmed our hypothesis that the starch-flocculating ability of Leuc. citreum SJ-57 was directly associated with its surface proteins. However, these extracted proteins exhibited starch-flocculating ability with an FR of 28.89%, which was significantly lower than that of the same amount of intact bacterial cells (FR of 51.27%) (Fig. 4a). This result further confirmed the importance of intact cell structure to Leuc. citreum SJ-57's ability to flocculate starch.
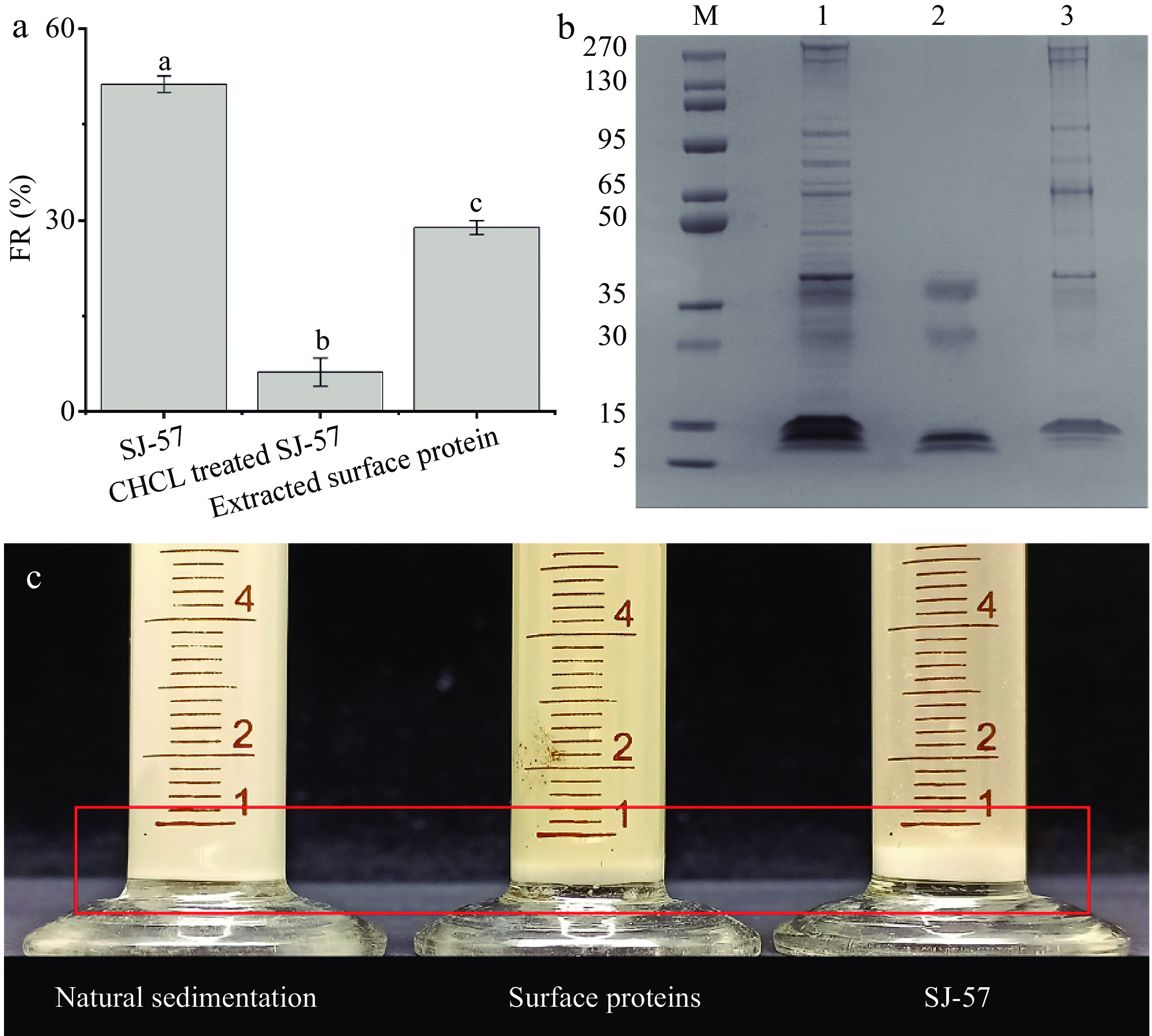
Figure 4.
(a) Effect of extraction reagents on the starch-flocculating activity of Leuc. citreum SJ-57. Different letters represent significant differences (p < 0.05). (b) SDS–PAGE analysis of whole cell surface proteins (lane 1), unbound starch of cell surface proteins (lane 2), and starch-binding surface proteins (lane 3). The molecular masses (in kDa) of the standard proteins (lane M) are indicated on the left. (c) Sedimentation of starch emulsions after resting for 5 min. Control: natural sedimentation of starch emulsions. Cell surface proteins: sedimentation of starch mixed with surface proteins. SJ-57: sedimentation of starch mixed with Leuc. citreum SJ-57.
In addition, we analyzed the electrophoretic bands of the cell surface proteins before and after starch flocculation, shown in Fig. 4b. The surface proteins after the reaction (lane 2) had a significantly lower number of bands compared to the surface protein profile before the reaction with starch (lane 1). Meanwhile, the starch granules were boiled directly after binding the surface protein, so we obtained the surface protein that binds to the starch (lane 3). It is clear that bands in lane 1 are the sum of bands in lane 2 and lane 3 as they are complementary to each other (Fig. 4b). This result provided further evidence that the cell surface proteins of Leuc. citreum SJ-57 indeed could bind with starch granules. It is in great agreement with the previous study of LAB, which showed that the starch-flocculating activities of L. paracasei L1 and Streptococcus lactis were the work of their cell surface proteins[3, 34]. In addition, starch-adhesive LAB, such as Bifidobacteria and L. amylovorus, would lose their ability to attach to starch when cell surface proteins were removed[11, 13, 15].
The starch-binding proteins in Fig. 4b were further identified using LC-ESI-MS/MS. The candidate proteins with the highest score for each band were listed in Table 3, and most of them were known to have adhesin functions. The isoelectric points of the bound starch proteins we identified ranged from 4.60 to 5.26, implying that they were all negatively charged in a neutral environment. The proteins with an MW of ~280, ~270, and ~95 were identified as GW-domain-containing proteins, which could non-covalently attach to the cell wall of Gram-positive bacteria[36]. Their starch-binding property might result from glucan-binding domains in their structure[37]. The proteins with an MW of ~65 and ~50 were identified as molecular chaperone DnaK and chaperonin GroEL, respectively. Both proteins were molecular chaperones with roles to assist in folding newly synthesized or unfolded polypeptides and pathogen adhesion[38]. The protein with an MW of ~40 was identified as elongation factor Tu (EF-Tu). In addition to its presence in bacteria's cytoplasm, EF-Tu had been found in other cellular compartments, hence its association with the cell wall of Leuc. citreum SJ-57 was not surprising[39]. It was demonstrated to assist in the adhesion of probiotic bacteria[40], so EF-Tu is highly likely the major contributor to Leuc. citreum SJ-57's high adhesion capacity. The last two bands were identified as lysozyme M1, which participated in the growth, turnover, and maintenance of the cell wall and the separation of daughter cells[3]. Similarly, lysozyme M1 was also found on the surface of the starch-adhesive LAB L. paracasei L1[3]. Overexpression of proteins involved in bacterial adhesion was observed in the cell wall proteome of the highly adhesive strain L. plantarum WHE 92, including elongation factor EF-Tu, GroEL chaperonin, molecular chaperone DnaK, and Lysozyme M1[41]. Due to extensive functional overlap between proteins, they may all participate in the starch binding and flocculating of Leuc. citreum SJ-57. The expression of these proteins could be further explored in future studies. In addition, it was also found that Leuc. citreum SJ-57 could only flocculate starch granules in the presence of metal ions but not in pure DI water (Fig. 1c). Hence, such ionic-strength-dependent interaction enabled us to deduce that those cell surface proteins might interact with starch granules via metal ions.
Table 3. Candidate starch-binding proteins extracted from the surface of Leuc. citreum SJ-57.
Band Protein Accession
numberNumber of
matchesScore Isoelectric
pointMolecular
weight (KD)1 GW (glycine-tryptophan)) dipeptide domain-containing WP_146992371.1 154 3546 5.26 230 2 GW (glycine-tryptophan)) dipeptide domain-containing WP_146992371.1 867 22986 5.26 230 3 GW (glycine-tryptophan)) dipeptide domain-containing WP_146992371.1 761 18792 5.26 230 4 molecular chaperone DnaK WP_040177072.1 281 6808 4.60 65 5 Chaperonin GroEL WP_004901547.1 1085 28391 4.60 57 6 Elongation factor Tu WP_004900335.1 297 6485 4.82 43 7 Lysozyme M1 WP_004901157.1 974 26689 5.08 36 8 Lysozyme M1 WP_004901157.1 110 2473 5.08 36 Contribution of EPS and cell wall to flocculation capacity
-
The result of dextranase-treated cells suggested EPS on the surface of Leuc. citreum SJ-57 inhibited its starch flocculation ability (Fig. 3b). The EPS of Leuconostoc was more readily eluted with NaCl than other components covalently or non-covalently bound to the cell surface[35]. To exclude the effect of loss of cell surface proteins, we designed a time gradient for eluting the cells using NaCl solution while total sugar and protein content in the eluates were simultaneously determined. It could be seen that the FR of bacteria gradually increased in the first 20 min before it began to decline (Fig. 5a). In the meantime, Fig. 5b shows that the protein was undetectable in the eluate solution until 20 min, whereas the total sugar content in the wash solution was continuously increasing. Combining these results, we can deduce that the increase of Leuc. citreum SJ-57's starch-flocculating activity during the first 20 min was correlated with the removal of EPS from the cell surface. After 20 min, the starch-binding proteins started to detach from the cell, resulting in an inevitable decrease in FR. We then performed SDS-PAGE electrophoresis and flocculation reaction experiments on the eluate. The protein bands in the eluate were found to be identical with those previously extracted with GHCl (Supplemental Fig. S2), and these proteins showed apparent starch-flocculating ability.

Figure 5.
(a) Changes in FR as a function of time when Leuc. citreum SJ-57 cells were washed with NaCl. (b) The protein and EPS content changes in the wash solution as a function of time when Leuc. citreum SJ-57 cells were washed with NaCl. TEM pictures of Leuc. citreum SJ-57 (c) before and (d) after washing with NaCl for 6 h.
Based on the results of the enzyme treatment and the above experiments, we concluded that the EPS on the surface of Leuc. citreum SJ-57 could act as a shield for the cell surface proteins. EPS could influence bacterial aggregation, biofilm formation, adhesion, and survival[42]. The EPS envelope was demonstrated to interfere with bacterial adhesion in L. rhamnosus PEN and L. paraplantarum BGCG11[42, 43]. The EPS produced by Leuc. citreum was mainly dextran, which had a much larger molecular weight than the cell surface proteins[44]. In fact, the EPS of Leuc. citreum SJ-57 could be observed in a dendritic pattern around the cell wall and extracellular surface in Fig. 5c. Although the EPS of Leuc. citreum SJ-57 did not interact with the starch granules, it obscured the contact between the surface proteins and the starch granules, causing a decreased FR.
Contribution of the cell wall to starch flocculation capacity
-
To determine the function of the cell wall, we washed Leuc. citreum SJ-57 cells with saline for 6 h. The saline-washed cells presented a very smooth cell wall with little to no EPS or proteins (Fig. 5d). Such washed cells completely lost their ability to flocculate starch granules, which meant that the cell wall itself did not possess any starch-flocculating abilities. Above, we found that lysozyme treatment would substantially decrease the FR of Leuc. citreum SJ-57 as it could cause the total breakage of the cell wall, although the total amount of starch-flocculating proteins remained the same before and after the treatment. In the meantime, the extracted surface proteins could only cause apparent starch flocculation after 1 h, whereas the intact bacteria cells only needed a few dozen seconds to produce a similar effect. All these results implied that the presence of intact cell walls might facilitate the flocculation of starch granules by Leuc. citreum SJ-57.
We observed that the average particle size of the surface proteins was approximately 15 nm (data not shown). According to Fig. 2c, there was still more than 5 × 10−18 J repulsive energy between starch particles at 15 nm. The average particle size of Leuc. citreum SJ-57 was around 900 nm. Hence, there was almost no repulsive energy between particles at that distance. The extracted surface proteins had a substantially smaller particle size than bacteria cells, so they would need to overcome more repulsive energy between two starch granules to bind them. Therefore, the intact cell structure was necessary for SJ-57 to flocculate starch granules more efficiently.
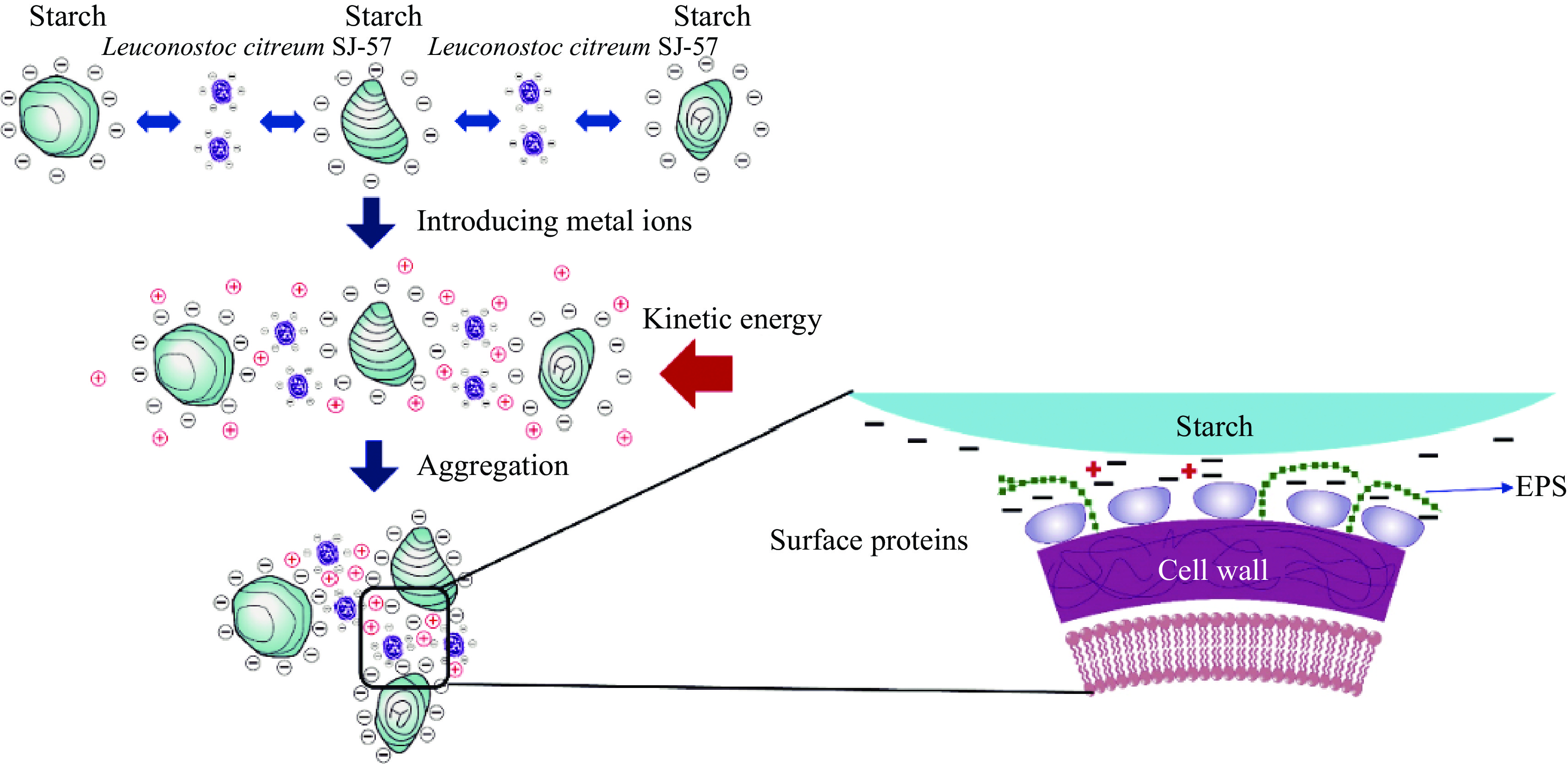
Based on all the evidence stated above, we proposed a possible starch flocculation mechanism of Leuc. citreum SJ-57, as illustrated in Fig. 6. First, the bacteria cells must cross the energy barrier and make contact with starch granules in the presence of external kinetic energy to initiate the flocculation process, i.e., shaking or stirring. The -COOH groups of the bacterial surface proteins formed ionic bonds with the metal cations in solution, i.e., Ca2+, K+, and Na+, which also bound with -OH on the starch granules via bridging action. The multiple surface proteins on the bacteria can capture several starch granules simultaneously and act as bridges between them, forming a floc. As a result, the starch granules accumulated via the volume-sweeping mechanism and gradually increased in size, leading to accelerated sedimentation. Cell surface proteins were critical to the strain's starch flocculation function, as they are the primary binding sites. However, the EPS existing on the surface could shield these active binding sites and compromise the flocculation process. Additionally, intact bacteria cells showed higher FR than the bacterial cell proteins alone, which could be explained that the large size of the bacterial cell enabled them to overcome the spatial repulsion between the starch granules.
-
In this study, the primary mechanism of how Leuc. citreum SJ-57 flocculate starch was proposed after thoroughly investigating the short-range and long-range interactions between bacteria and starch granules. Essential bacteria surface proteins interacting with starch through simultaneous ionic bonds with metal cations were identified, i.e., GW structural domain proteins, DnaK, GroEL, elongation factor Tu, and lysozyme M1. The negative effect of EPS and the positive effect of the intact bacterial cell on the flocculation rate were explained. This study provided a deeper understanding of the mechanism of microbiological flocculants and a foundation for their future industrial application in starch production.
-
The authors confirm contribution to the paper as follows: study conception and design: Liao X, Wu X, Rao L, Wang X; data collection: Wang X; analysis and interpretation of results: Wang X; draft manuscript preparation: Wang X, Osei P. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
This work was supported by Sichuan Science and Technology Program 2023NSFSC0181 and the 2115 Talent Development Program of China Agricultural University.
-
The authors declare that they have no conflict of interest. Xiaojun Liao is the Editorial Board member of Food Innovation and Advances who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research groups.
- Supplemental Fig. S1 Effect of different pH on the FR of Leuconostoc citreum SJ 57.
- Supplemental Fig. S2 SDS-PAGE analysis of whole cell surface proteins (lane 1) and proteins in the eluent (lane 2). The molecular masses (in kDa) of the standard proteins (lane M) were indicated on the left.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang X, Osei PO, Rao L, Wu X, Liao X. 2023. Characteristics and mechanism of Leuconostoc citreum as a novel bioflocculant for starch granules in starch production. Food Innovation and Advances 2(4):291−301 doi: 10.48130/FIA-2023-0030
Characteristics and mechanism of Leuconostoc citreum as a novel bioflocculant for starch granules in starch production
- Received: 14 August 2023
- Accepted: 23 October 2023
- Published online: 30 November 2023
Abstract: The Leuconostoc citreum SJ-57 strain isolated from the sweet potato starch production showed great potential as a microbiological flocculant, but its underlying flocculation mechanisms are yet unknown. In this study, infrared spectroscopy and thermodynamic analysis were performed to elucidate the short-range and long-range interactions between Leuc. citreum SJ-57 and starch granules, revealing that bacteria cells bond starch granules via metal-bridging ionic bonds. A high repulsive energy barrier of ~8 × 10−18 J must be overcome to initiate the flocculation process. Heat, protease, lipase, lysozyme, dextranase, and guanidine hydrochloride were used to treat the bacterial cell, confirming that its flocculation ability originated from surface proteins, including GW structural domain proteins, DnaK, GroEL, elongation factor Tu, and lysozyme M1. The primary flocculation mechanisms of Leuc. citreum was proposed to provide a deep understanding of microbiological flocculants and a foundation for future industrial applications in starch production.