-
Aronia melanocarpa, also known as black chokeberry, is a deciduous perennial shrub of the Rosaceae family native to Eastern North America. Its fruits are purple-black in color and rich in phenolic substances, such as anthocyanins, phenolic acids, and flavonoids, especially anthocyanins, up to 686 mg/100 g fresh weight, which is the highest content of any known plant[1]. Studies have shown that Aronia melanocarpa has strong antioxidant, antibacterial, anti-inflammatory[2,3] and hypotensive[4] effects, and its antioxidant activity is much higher than that of blueberries and cranberries. Aronia melanocarpa has been described as the third generation of the 'fruit of the future', with strong healthy and medicinal values. The taste of Aronia melanocarpa is sour and difficult for consumers to accept; therefore, it is usually processed into juice, wine, and dried fruit[5].
Juice is a common deep-processed product of Aronia melanocarpa. Sterilization is the most significant processing step in juice production and the sterilization method and parameters directly affect the organoleptic quality and nutritional properties of juice products. The efficacy of sterilization and its influence on the overall quality of juice largely depend on the sterilization method and parameters, microbial species, and the juice matrix[6]. Therefore, it is important to explore the influences of different sterilization techniques on the overall quality of different types of juices to produce high-quality juices. Currently, most juice processing methods are based on traditional thermal sterilization. Ultra-high-temperature instantaneous sterilization (UHT) is a kind of widely used thermal sterilization techniques in the industry today and is widely used in the manufacture of liquid foods, such as dairy and fruit juices, at a relatively low cost. However, high temperatures inevitably have some negative effects, which cause deterioration of color and flavor, changes in physicochemical properties, and reduction of heat-sensitive nutritional and functional components in the juice, and may form 5-HMF and furan, which can be detrimental to the health of consumers[6,7]. Over the last 20 years, a series of non-thermal sterilization methods, including high hydrostatic pressure sterilization (HHP), ultrasound sterilization, cold plasma sterilization, and irradiation sterilization (IS), have been widely used to sterilize various types of juices. The influences of different sterilization methods on the physicochemical properties, organoleptic characteristics, nutritional composition, and functional activity of juice products have been investigated. HHP is the only non-thermal sterilization technique that has been successfully applied at juice industrial production. It mainly destroys microbial cells and enzyme structures at high pressure to obtain a longer shelf life of juices. IS mainly acts directly or indirectly on biological macromolecules, such as proteins, lipids, and nucleic acids, through high-energy rays to change their biochemical properties, destroy their structures, and reduce or lose their biological functions, thereby killing microorganisms. Foods irradiated at doses below 10 kGy have been reported to be safe[8], and IS treatment is currently effective for sour cherry juice[9], tamarind juice[10], and carrot juice[11]. Hurdle technologies typically exhibit better sterilization effects than single-sterilization methods[6]. Ultrasonic treatment not only inactivates microorganisms and enzymes through a cavitation effect, but also promotes the extraction of bioactive substances from liquid foods. It is usually combined with mild heat treatment to achieve better sterilization and inactivated enzyme effects. Research have indicated that thermosonication (TS) can sustain the quality parameters of kutkura juice, orange juice, and blueberry juice[12−14].
Currently, most industrial sterilization methods for Aronia melanocarpa juice (AMJ) are based on thermal sterilization, which results in a large loss of nutritional and functional components, especially anthocyanins[15]. Therefore, there is an urgent need to systematically compare the influences of different sterilizations on the quality of AMJ and select suitable sterilization methods for processing high-quality AMJ.
Therefore, this study took anthocyanin-rich AMJ as the research object to explore the influences of one conventional thermal sterilization (UHT), two non-thermal sterilization methods (HHP, IS) and one hurdle technology (TS) on the quality attributes of AMJ. Anthocyanin-targeted metabolomics was used to explore the influences of diverse sterilization methods on the AMJ anthocyanin profile. The correlation between the antioxidant activity, color properties, and functional components of AMJ was also analyzed. The results of this study are expected to provide a theoretical foundation and technical parameter for the high-value nutritional processing of AMJ.
-
Aronia melanocarpa from Shennong Zhihua Biotechnology Co., Ltd. (Shanxi, China). Fresh berries are stored in polyethylene bags at −20 °C until juicing. All standards and chemicals including catechol (CA), cyanidin-3-glucoside (C3GE), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), gallic acid (GA), 2,4,6-tripyridyl-s-triazine (TPTZ), and so on were purchased from Sigma-Aldrich (St. Louis, USA). Pectinase and cellulase were purchased from Henan Wanbang Chemical Technology Co., Ltd. (Henan, China). All other agentia used in the assay were purchased from Aladdin Co., Ltd. (Shanghai, China). The standard products used are chromatographic pure, and the other reagents are analytically pure.
AMJ preparation
-
Aronia melanocarpa was thawed, washed, and pulped until no large pulp pieces were observed. The method used was a refinement of the method of Zhao et al.[16] & Xu et al.[17], AMJ was obtained by adding 0.02% (m/m) pectinase (50 U/mg) and 0.1% (m/m) cellulase (10 U/mg), enzymatically digested at 45 °C for 2 h. Then centrifuged under 7,000 g and 4 °C for 15 min to obtain AMJ (GL-10MD, Xiangyi Co., Ltd., Hunan, China). AMJ was transferred to a food-grade high-density polyethylene (HDPE) bucket and stored at 4 °C. All sterilizations were completed within 12 h.
Sterilization process
-
Unsterilized AMJ from the same batch of juice was used as the control check group (CK), and unsterilized AMJ was treated with UHT, TS, HHP, or IS. The UHT treatment was carried out at 130 °C for 5 s using the HzS jj50 ultra-high temperature instantaneous sterilization system (Shanghai Gexin Mechanical Technology Co., Ltd., Shanghai, China) and then aseptically filled and sealed in HDPE bottles[18]. TS treatment was carried out using an ATPIO-1000D ultrasonicator (Nanchang Xianou Co. Ltd., Jiangxi, China) with a 6 mm diameter horn microtip connected to a thermostatic water bath system, ultrasonic power of 700 W, frequency range of 20−25 kHz and pulse duration of 2 s on and 3 s off, treated at 55 °C for 10 min and then transferred to HDPE bottles in a sterile environment[18]. In the HHP group, the common parameters used in industry was selected. AMJ was subjected at 500 Mpa for 10 min at 25 °C using an HHP.L2-600 hydrostatic pressurization unit (Suyuan Zhongtian Co. Ltd., Beijing, China)[19]. In the IS group, AMJ were packed in transparent bottles up to 4 cm in height and subsequently irradiated at 3 kGy (Yang Ling HeSheng Irradiation Technology Co., Ltd., Shaanxi, China)[16,20]. The AMJ samples were tested for microbiological assays immediately after the treatments and the rest of the samples were stored at 4 °C and the other indicators were completed within 48 h.
Microbiological assays
-
Microbiological assays: total bacterial content (TBC), Escherichia coli (E.coli), and mold and yeast were carried out by the plate counting method and the most probable number (MPN) method according to the Chinese National Standards GB/T 4789.2-2016, GB/T 4789.3-2016, and GB/T 4789.15-2016, and the results were shown as CFU/mL and MPN/mL. The specific experimental operations referred to the method described by Ma et al.[21].
Physicochemical characteristics
-
The pH was carried out using a PHS-3E pH meter (Leici Co., Ltd., Shanghai, China). The total soluble solids values (TSS) were recorded using a PAL-1 digital Abbe Refractometer (ATAGO Co. Ltd, Tokyo, Japan) and shown as °Brix. The titratable acidity (TA) values were assayed by acid-base titration method based on the Chinese National Standards GB/T 12456-2008. The ratio of total soluble solids to titratable acidity (RTT) was expressed as TSS/TA. The browning index (BI) was evaluated following the method described by Yuan et al.[22]. The juice was diluted 20 times with an 80% ethanol solution (acidified with 1% hydrochloric acid), and the absorbance values (A420 and A510) were determined using a UV-2800 spectrophotometer (UNICO Co., Ltd., Shanghai, China) at 420 and 510 nm, and the BI values were expressed as A420/(A420 + A510). The viscosity was measured in mPa·s using a NDJ-8S rotational viscometer (Weiling Scientific Instruments Co., Ltd., Shanghai, China).
Functional characteristics
Measurement of total anthocyanins content (TAC), total polyphenols content (TPC) and total flavonoids content (TFC)
-
Referring to the methods of Bao et al.[18], the dual-wavelength pH-differential method, Folin-Ciocalteu method, and AlCl3 colorimetric method were used for the determination of TAC, TPC, and TFC. The results were shown as mg C3GE/L, mg gallic acid equivalents (GAE)/L, and mg catechin equivalents (CAE) /L.
Antioxidant capacity assays
-
Following the methods of Ma et al.[23] & Zulueta et al.[24], antioxidant capacity of AMJ was measured using three indexes: The 1,1-diphenyl-2-picryl-hydrazyl radical free radical scavenging activity (DPPH), ferric reducing antioxidant power (FRAP), and ABTS radical cation scavenging activity (ABTS). The colorimetric methods were used for the determination of all three indexes. ABTS solution was reacted away from light and left overnight to produce. The results are reported in mmol trolox/L.
Anthocyanin-targeted metabolomics
-
The method used was a refinement of the method of Bao et al.[25]. Aqueous 50% methanol (chromatographic grade), acidified with 0.1% hydrochloric acid, was used as the extraction solution. Nine mL of AMJ and 36 mL of extraction solution were added into a 50 mL centrifuge tube and extracted for 15 min under ultrasound at 30 °C, 40 W. After sonication, the extract was passed through 0.22 μm organic membranes. The UPLC-ESI-MS/MS system (ExionLCTM AD, Framingham, MA, USA; QTRAP®6500+, Foster City, CA, USA) was in use for data acquisition. The liquid phase conditions consisted of a Waters ACQUITY BEH C18 column (1.7 μm, 2.1 mm * 100 mm); mobile phases A and B were ultrapure water and methanol with the addition of 0.1% formic acid, respectively; elution gradient: 0.00−6.00 min, increasing phase B from 5% to 50%, 12.00 min to 95%, holding for 2 min and then decreasing to 5%, equilibration at a flow rate of 0.35 mL/min for 2 min with a column temperature of 40 °C. Injections volume were set to 2 μL. Samples in the positive ion mode were analyzed by a QTRAP® 6500 + mass spectrometer with an electrospray ion source (ESI) under the control of Analyst 1.6.3 software (Sciex). The ESI temperature was 550 °C. The mass spectrometry voltage for the positive ion mode was 5,500 V. Curtain gas pressure of 35 psi was used. Each ion pair was swept in Q-Trap 6500+ on the basis of optimised declustering potentials and collision energies.
Metware Database (MWDB) is constructed based on standard products, and qualitative analysis of mass spectrometry data is carried out. The metabolites were identified by comparing the m/z values, retention time and fragmentation patterns with the standards in the MWDB[26]. The quantitative detection of metabolites were performed using multiple reaction monitoring (MRM) in positive ion mode by the MetWare Biotechnology Co., Ltd. (Wuhan, China)[25]. The characteristic ions of metabolites were screened by QQQ mass spectrometer and the signal intensity was obtained. Integration and correction of chromatographic peaks were performed using MultiQuant 3.0.3 software. The corresponding relative metabolite contents were expressed by the chromatographic peak area integrals[27].
Color characteristics
-
The samples'color parameters including red-greenness (a*), yellow-blueness (b*), brightness (L*), hue (h), Chroma (C*), and total color differences (ΔE) were assessed and calculated by the Ci7600 colorimeter (X-rite, Michigan, USA) using full transmissive mode.
Statistical analysis
-
Data were analyzed and graphed with Excel 2016, SPSS 20, and Origin 2022. Unless otherwise stated, the results of the test are expressed as means ± standard deviation. All data were measured at least in triplicate. One-way ANOVA, Waller-Duncan's multiple range tests (p < 0.05), and significance analysis were performed using SPSS 20 software, when p < 0.05, implying a significant difference.
-
The results for TBC, E.coli, and mold and yeast in the juice after sterilizations are shown in Supplemental Table S1. Under the sterilization parameters used in this study, TBC, E.coli, and mold and yeast were not detected in AMJ after the four sterilizations, proving that all sterilization treatments in this experiment could efficiently guarantee the microbiological safety of AMJ.
Influences of sterilization treatments on physicochemical indexes
-
The influences of the diverse sterilization methods on the physicochemical indexes of AMJ were presented in Fig. 1. Figure 1a showed that the pH variation of AMJ after HHP and IS treatments were not significant compared with that of the CK group (p > 0.05), whereas UHT and TS treatments caused a significant increase (p < 0.05). It was found that HHP treatment might cause the free acid in the juice to form a buffer substance with inorganic salts and other substances[28], preventing significant changes in pH. Similarly, IS treatment at 3 kGy had no obvious influence on the pH of AMJ (p > 0.05), whereas Naresh et al.[20] found the pH of mango juice irradiated with 3 kGy increased significantly, which could be due to the different juice matrices. For TA, another important indicator of juice acidity, the IS treatment did not significantly change TA in the juice (p > 0.05), while the other three sterilization methods significantly reduced TA in the juice (p < 0.05). The UHT and IS treatments declined the TSS in the AMJ significantly (p < 0.05), with the lowest TSS of 15.7 °Brix in the UHT-treated juice (Fig. 1b). This was probably due to the high-temperature treatment causing some of the soluble materials to precipitate as flocculent precipitates[29]. The reasons for the decrease in TSS levels caused by IS treatment require further investigation. RTT is an important indicator of the sweet and sour palatability of juices, which affects their organoleptic acceptability and consumer preference. The RTT of CK group was only 14.54 (Fig. 1b) and its taste was relatively sour; therefore, a higher RTT would make it more popular among consumers[15]. All treatments except IS significantly increased the RTT of AMJ, with the highest RTT of 15.49 after TS treatment, indicating that TS-treated juice was more likely to be preferred by consumers in terms of sweetness and acidity suitability.
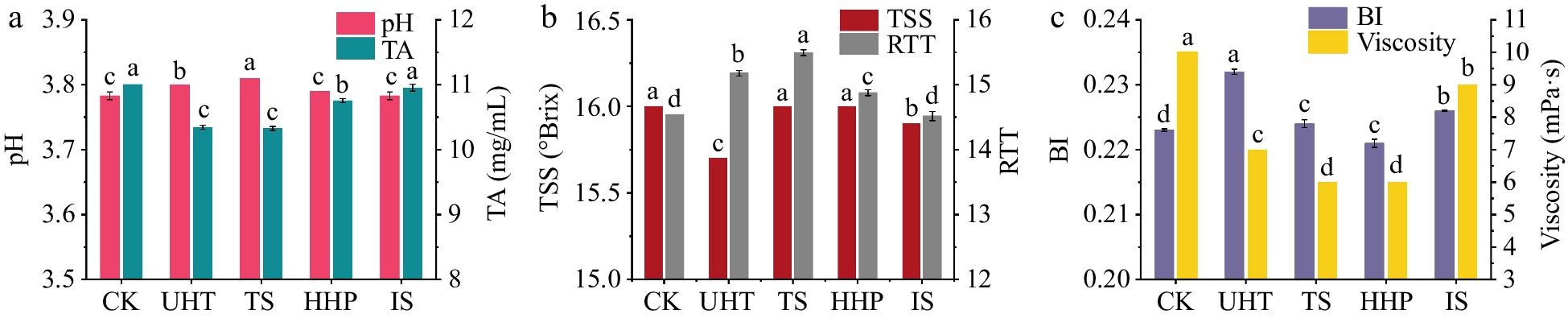
Figure 1.
Influences of various sterilization methods on the physicochemical indexes of AMJ. (a) pH and TA; (b) TSS and RTT; (c) BI and viscosity. Different lowercase letters in the figure indicate significant differences (p < 0.05).
As shown in Fig. 1c, in comparison to the CK group, the HHP treatment caused a markedly decline in BI, whereas the others led to a significant increase in BI (p < 0.05). Although the different sterilization treatments could inactivate polyphenol oxidase and peroxidase, which caused enzymatic browning of the juice to some extent, UHT, TS, and IS inevitably exacerbated non-enzymatic browning of the juice, leading to a obvious increase in BI values[22,30]. Viscosity is an important physicochemical indicator of juice, and a reduction in viscosity is not conducive to the homogeneity and cloudy stability of juice, leading to its precipitation[31]. All sterilization treatments significantly reduced the juice viscosity (p < 0.05). Among them, the HHP and TS treatments had the greatest effect on AMJ's viscosity. The influence of TS treatment on AMJ's viscosity might be due to the high energy of the ultrasonic wave that destroyed molecules of pectin and reduced the carbohydrate molecular weight, thus reducing the viscosity of AMJ[32]. Generally, due to the presence of pectin methylesterase, the viscosity of juice after HHP treatment tends to stabilize or increase[17]. In this experiment, the decrease of AMJ's viscosity after HHP treatment might be attributed to disruption of noncovalent bond between the remaining pectin molecules and other components under high pressure, and the same conclusion was obtained in HHP-treated cloudy strawberry juice[33]. Therefore, the effect of HHP treatment on the viscosity of juice needs to be further studied.
Influences of sterilization methods on the functional characteristics
-
Polyphenols such as anthocyanins and flavonoids are a category of secondary metabolites with high biological activity and are important base materials for the functional activity of Aronia melanocarpa[34]. The influences of different sterilization methods on TAC, TPC, and TFC in AMJ are presented in Fig. 2a−c. The TAC, TPC and TFC in the AMJ of the CK group were 736.33 mg C3GE/L, 4285.09 mg GAE/L and 2,227.78 mg CAE/L, respectively. TS treatment significantly increased TAC, TPC, and TFC by 5.27%, 2.47%, and 10.91%, respectively (p < 0.05), mainly because TS treatment disrupted the cell walls and vacuoles in plant tissues, and the ultrasonic cavitation caused cavitation collapse in surrounding colloidal particles, which facilitated the release of more phenolic compounds into the juice, thus increasing the TAC, TPC, and TFC in the juice[35]. Similarly, TAC and TFC significantly increased by 2.01% and 4.68%, respectively, after HHP treatment (p < 0.05), which accelerated mass transfer by disrupting cell integrity and increasing membrane permeability to release phenolics present in plant vesicles, thereby increasing their extractability and extraction rate in the juice[36,37], and similar findings were obtained in HHP-treated NFC spine grape juice[38]. The loss of TAC, TPC and TFC after UHT treatment was 8.79%, 5.12%, and 17.14% respectively, indicating that anthocyanins and flavonoids were more sensitive to temperature, high temperature of 90−120 °C has been reported to cause chemical oxidation of phenolic substances to form quinones and their polymers and hydrolysis of esters and glycosides, leading to the phenolic compounds being degraded[39]. The significant decrease in functional substances in the AMJ after IS treatment was due to the degradation of photosensitive components (p < 0.05)[6]. Alighourchi et al.[8] also discovered a significant decline in the TAC of irradiated pomegranate juice; however, other studies showed that irradiation did not cause a reduction in functional substances in juices, such as irradiated ashitaba and kale juices[40] and sour cherry juice[9]. This is mainly because the relative stability of anthocyanins in juices relies on several elements, like the juice matrix, structural characteristics, and processing conditions.
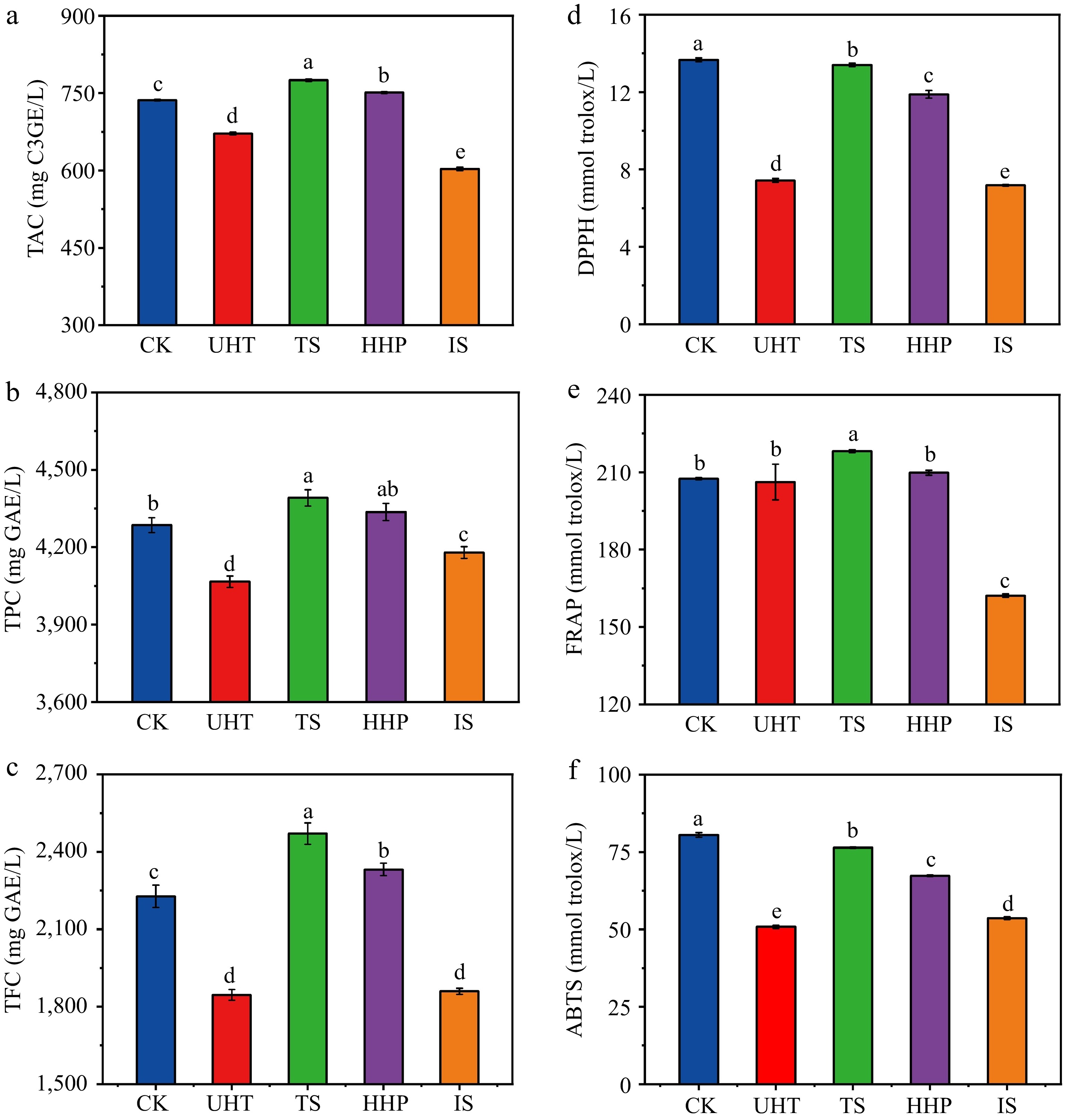
Figure 2.
Effects of different sterilization methods on functional indexes and antioxidant capacities of AMJ. (a) TAC; (b) TPC; (c) TFC; (d) DPPH; (e) FRAP; (f) ABTS. Different lowercase letters express significant differences (p < 0.05).
The results of the different sterilization treatments on AMJ's antioxidant capacity are shown in Fig. 2d−f. The DPPH of the CK group was 13.67 mmol trolox/L, which was markedly higher than that of sterilized juice (p < 0.05), indicating that all sterilization treatments reduced the DPPH in the juice. The TS and HHP groups retained 98.02% and 86.91% of the DPPH, respectively, whereas the UHT and IS groups retained only 54.40% and 52.56%, respectively. In Fig. 2e, FRAP in the CK was 207.51 mmol trolox/L, which was significantly enhanced by 5.15% after TS treatment and significantly decreased by 21.86% after IS treatment (p < 0.05). Fig. 2f shows that all sterilization methods caused ABTS decrease significantly (p < 0.05). The decrease in the TS group was only 5.10%, followed by the HHP group, whereas the decrease in the UHT group was as high as 36.88%. Combining the results of the three antioxidant assays, TS treatment was the most beneficial for the retention of antioxidant activity in AMJ, and similar findings were obtained in TS-treated peach juice[41]. HHP treatment was followed only by TS treatment in terms of retention of antioxidant capacity in the juice (Fig. 2a−c), mainly because both the two sterilization methods could significantly enhance or retain the functional substances in AMJ. Numerous studies have shown that the antioxidant capacity of juices is significantly positively related to the levels of functional substances[42]. Therefore, the IS treatment performed poorly in the three different antioxidant evaluation systems. Arjeh et al.[9] also observed a remarkable reduction of antioxidant capacity in irradiated sour cherry juice.
Analysis of anthocyanin metabolites
-
To clarify the changes in anthocyanin metabolites after different sterilization treatments, the characteristic functional component of AMJ, anthocyanin-targeted metabolomics, was performed using UPLC-MS/MS on five groups of juice samples. Figure 3a shows a standardized PCA score chart based on the results of anthocyanin-targeted metabolomics. PC1 and PC2 together explained 88% of the total variance, indicating that PCA could better explain the results of anthocyanin-targeted metabolomics data. In the PCA score chart, the IS, UHT, and CK groups were clearly separated from the remaining groups, whereas the TS and HHP samples were not well separated, indicating that the anthocyanin profiles of the TS and HHP groups were similar overall, while the other groups differed considerably. The total monomeric anthocyanins contents (TMAC) in AMJ after different sterilization treatments was presented in Fig. 3b. The TMAC of the CK group was found to be 991.43 mg/L, and TS treatment significantly enhanced the TMAC in the AMJ, whereas IS treatment significantly reduced the TMAC (p < 0.05). Overall, the trend of TMAC measured by anthocyanin-targeted metabolomics was consistent with that of the TAC measured above. In terms of anthocyanin monomers, Cyanidin-3-O-galactoside (C-3-O-gal) and Cyanidin-3-O-arabinoside (C-3-O-ara) were relatively high-content anthocyanin monomers in AMJ, accounting for 76% and 11% of TMAC, respectively. In addition, Cyanidin-3-O-xyloside (C-3-O-xyl), Cyanidin-3-O-glucoside (C-3-O-glu), and rutin accounted for 8%−9% of the TMAC.
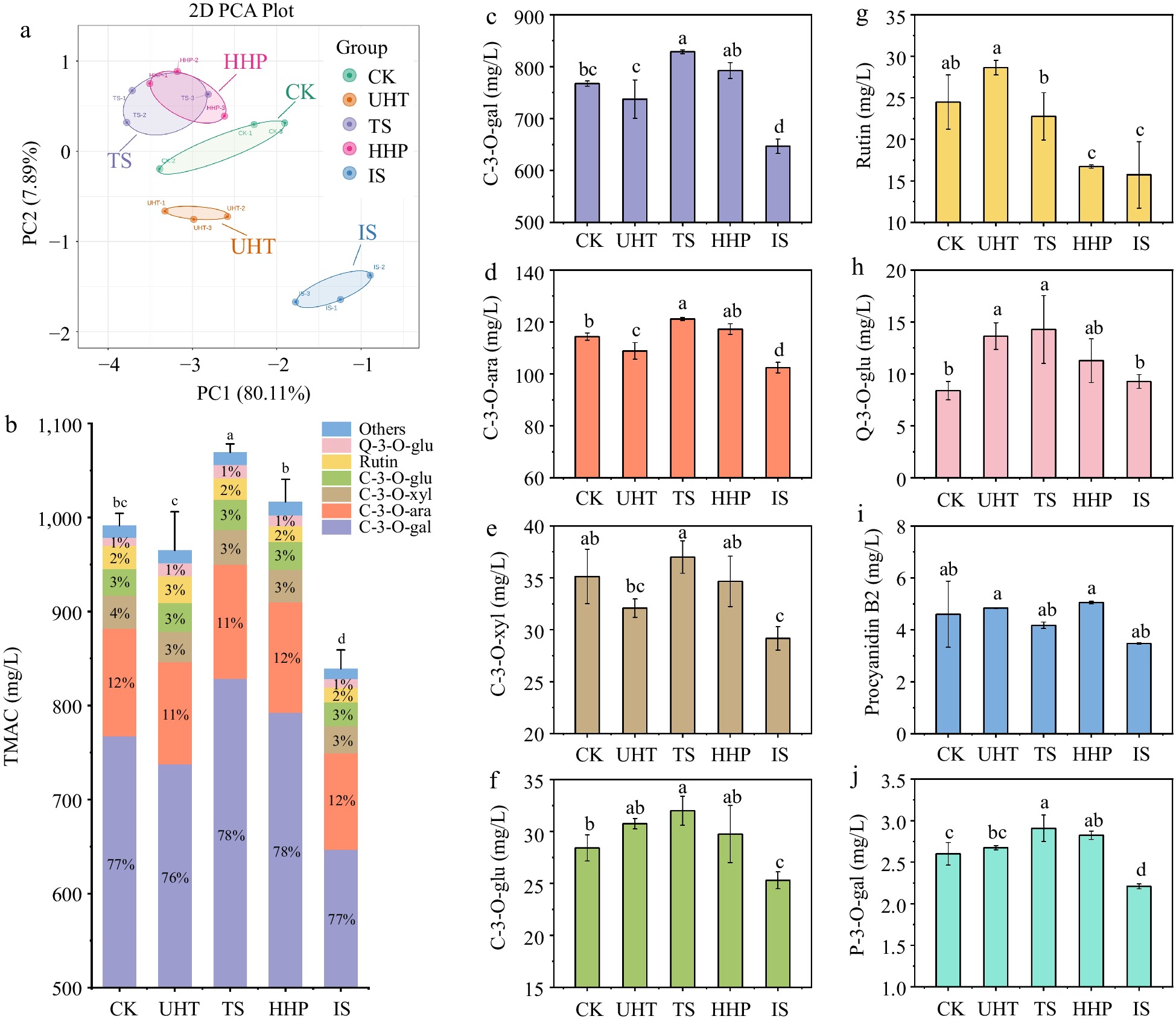
Figure 3.
(a) The PCA score map, (b) TMAC, and (c)–(j) main anthocyanin monomer (> 2.5 mg/L) content of AMJ. Different lowercase letters express significant differences (p < 0.05).
Figure 3c−j show the contents of the eight major anthocyanin monomers (> 2.5 mg/L) in the AMJ. As shown in Fig. 3c & d, C-3-O-gal and C-3-O-ara contents in CK were 767.50 mg/L and 114.38 mg/L, respectively, TS treatment significantly increased C-3-O-gal and C-3-O-ara contents in AMJ by 7.98% and 5.90%, while IS resulted in a significant decrease of 15.74% and 10.46% (p < 0.05). The contents of C-3-O-gal and C-3-O-ara accounted for 88.95% of TMAC in CK group, and the change of their contents had a great influence on TMAC. Thus, C-3-O-gal and C-3-O-ara were the principal reasons for the significant changes in TMAC after the TS and IS treatments. After UHT and HHP treatments, only the UHT group showed a significant reduction in the content of C-3-O-ara.
Figure 3e−j show that among the six substances, UHT treatment only caused a significant increase in Quercetin-3-O-glucoside (Q-3-O-glu) (p < 0.05), whereas the contents of the remaining five monomers did not change significantly (p > 0.05). This may be because thermal treatment led to the degradation of phenolics, at the same time, it also disrupted the cell wall and cell membrane structure, thereby enhancing the release of some phenolic compounds[43]. After TS treatment, except for the contents of C-3-O-xyl, rutin, and procyanidin B2, which showed no significant changes (p > 0.05), the other three anthocyanins showed a noticeable increase. Thus, TS treatment could retain or significantly increase the major anthocyanins in AMJ. The Pelargonidin-3-O-galactoside (P-3-O-gal) was significantly up-regulated while rutin showed significant down-regulation after HHP treatment (p < 0.05), and a reduction in rutin content was also found in HHP-treated tomato pulp[44]. IS treatment caused a significant reduction in the major anthocyanin monomers in AMJ, except for Q-3-O-glu and procyanidin B2 (p < 0.05), suggesting that apart from these two substances, the other four were sensitive to IS treatment to some extent.
In addition, the differential metabolites of all anthocyanin monomers in AMJ were discussed (Fig. 4a−j). Differential metabolites were screened for significant differences based on log2 FC < −1 or > 1. All treatments resulted in significant reductions in Cyanidin-3-O-sambubioside (C-3-O-sam) and chalcone, and chalcone was not detected after TS, HHP, and IS treatments (Fig. 4a, f & g), indicating that C-3-O-sam and chalcone may be marker differential metabolites in the CK and that the two anthocyanin monomers were extremely unstable in AMJ and susceptible to different sterilization methods. Malvidin-3-O-glucoside (M-3-O-glu) was significantly elevated after TS treatment compared to the other four groups, and the log2 FC of CK and TS group reached 1.40991; thus, M-3-O-glu may be a marker differential metabolite produced by TS treatment (Fig. 4h). Cyanidin-3-O-(6-O-malonyl-β-D-glucoside) was significantly lower in HHP-treated juice than that of the other four groups (p < 0.05), suggesting that it was more sensitive to pressure and HHP might cause breaks in its chemical bonds, thus changing or decomposing its structure[45]. Therefore, it may be a marker of differential metabolites produced by HHP treatment (Fig. 4i). Although the content of Kaempferol-3-O-rutinoside (K-3-O-rut) after IS treatment was markedly lower than that in the other four groups, it was suggested that K-3-O-rut is extremely sensitive to irradiation and might be a characteristic marker produced in AMJ after IS treatment (Fig. 4j). It is worth noting that although chalcone also showed significant differences in the UHT group compared with the other groups (Fig. 4b & g), the level of chalcone was significantly lower in UHT group than in the CK, whereas it was markedly higher in UHT group than in TS, HHP, and IS groups; therefore, chalcone could not be used as a marker differential metabolite in the UHT group. Although few differential metabolites were screened among the different treatments, the marker differential metabolites might provide a reference basis for selecting and judging sterilization treatments for AMJ.

Figure 4.
Differential metabolites analysis. (a) Venn diagrams of differential anthocyanin metabolites from CK compared to other groups; (b) Venn diagrams of differential anthocyanin metabolites from UHT compared to other groups; (c) Venn diagrams of differential anthocyanin metabolites from TS compared to other groups; (d) Venn diagrams of differential anthocyanin metabolites from HHP compared to other groups; (e) Venn diagrams of differential anthocyanin metabolites from IS compared to other groups; and the content and structure maps of the marker differential anthocyanin metabolites produced by the (f), (g) CK, (h) TS, (i) HHP, and (j) IS treatments. Log2FC indicates the differential multiplicity of metabolites as a logarithmic result of a base value of 2. '*' expresses significant correlation (p < 0.05).
Correlation analysis of antioxidant capacity with anthocyanin monomers
-
To investigate the relationship between antioxidant capacity and functional components, such as anthocyanins, correlation analysis was performed between DPPH, FRAP, and ABTS and the contents of TAC, TPC, TFC, TMAC, and all detected anthocyanin monomers, as shown in Fig. 5. A strong correlation was considered to exist between the two when the Pearson correlation coefficient |R| was > 0.7. Among the antioxidant indicators, both DPPH and ABTS showed significant positive correlations with the contents of the three functional substances measured (DPPH: RTFC = 0.92, RTAC = 0.90, RTPC = 0.85; ABTS: RTFC = 0.86, RTPC = 0.82, RTAC = 0.81) (p < 0.05). However, FRAP was only significantly positive related with TAC (R = 0.90, p < 0.05). This showed that TFC, TAC, and TPC were the major contributors to the DPPH and ABTS of AMJ and that TAC was the main contributor to the FRAP of AMJ, which was similar to previous findings[46]. Among the anthocyanin monomers, all three antioxidant capacities DPPH, ABTS, and FRAP were significantly positive related with C-3-O-xyl (RDPPH = 0.80, RFRAP = 0.78, RABTS = 0.74), C-3-O-ara (RDPPH = 0.85, RFRAP = 0.86, RABTS = 0.76) and Pelargonidin-3-O-arabinoside (P-3-O-ara) (RDPPH = 0.82, RFRAP = 0.82, RABTS = 0.72) (p < 0.05). Thus, C-3-O-xyl, C-3-O-ara, and P-3-O-ara are thought to be the principal contributors to the antioxidant capacity of AMJ.
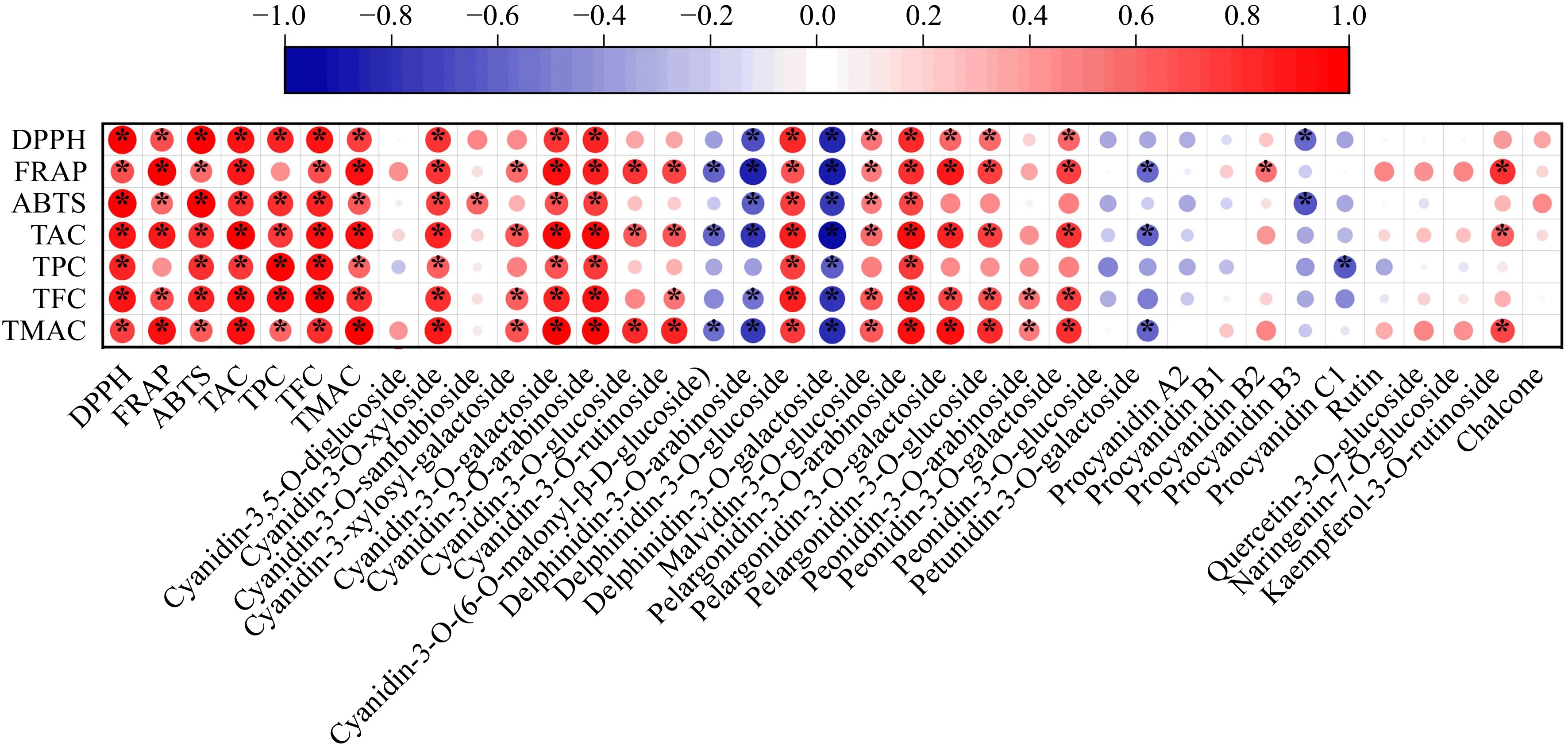
Figure 5.
Heatmap of correlation between DPPH, FRAP, ABTS, TAC, TPC, TFC, TMAC and each detected anthocyanin monomers in AMJ. '*' expresses significant correlation (p < 0.05).
Effects of sterilization methods on juice color
-
For AMJ, which is rich in anthocyanins and is mainly colored by anthocyanins, anthocyanins are susceptible to oxidation or degradation under the influence of different treatments, such as heating and irradiation; therefore, different sterilization treatments can significantly affect the color characteristics and sensory quality of juice. Therefore, it is crucial to study the influences of various sterilization methods on the color features of AMJ.
The influences of different sterilization methods on the color characteristics of AMJ are shown in Fig. 6a−e. When ∆E > 3, the juice color could be differentiated by the naked eye[18], combined with Fig. 6e, it can be seen that only the juices treated with UHT and IS showed visible changes that could be observed by the naked eye, while the color changes in TS and HHP groups were not significant. The a* and b* values reflect redness and yellowness, the C* and L* values reflect saturation and brightness, and h indicates the hue angle of the AMJ. The a* of the UHT-treated juice decreased from 24.61 to 18.77, and the C* value from 25.85 to 19.63 (Fig. 6a & c, p < 0.05). In addition to the color deterioration caused by the loss of anthocyanins, severe browning of the juice at high temperatures was another reason for the color change[47], which was in accordance with the findings of the BI values determined above. IS treatment significantly changed the color of the AMJ to redder and yellower (a* = 31.98, b* = 11.92), fuller (C* = 34.13), and brighter (L* = 7.33) (p < 0.05). The elevated L* and b* of the juice may be due to the partial loss of anthocyanins as a result of irradiation[15,48]. However, there is no reasonable explanation for the increase in a* values of juices after irradiation, Naresh et al.[20] and Shahbaz et al.[49] also found similar phenomena in irradiated mango juice and pomegranate juice, so it was speculated that this may be caused by other non-anthocyanin substances related to coloration produced during irradiation, which still needs further study. The a*, C*, L* and h values of AMJ after TS and HHP treatments were not significantly changed compared to CK (Fig. 6a−d, p < 0.05), and the ∆E were only 0.35 and 0.66 (Fig. 6e), therefore, the color of AMJ after TS and HHP treatments was most similar to the color of the CK group and that the natural color quality of the AMJ was best preserved.
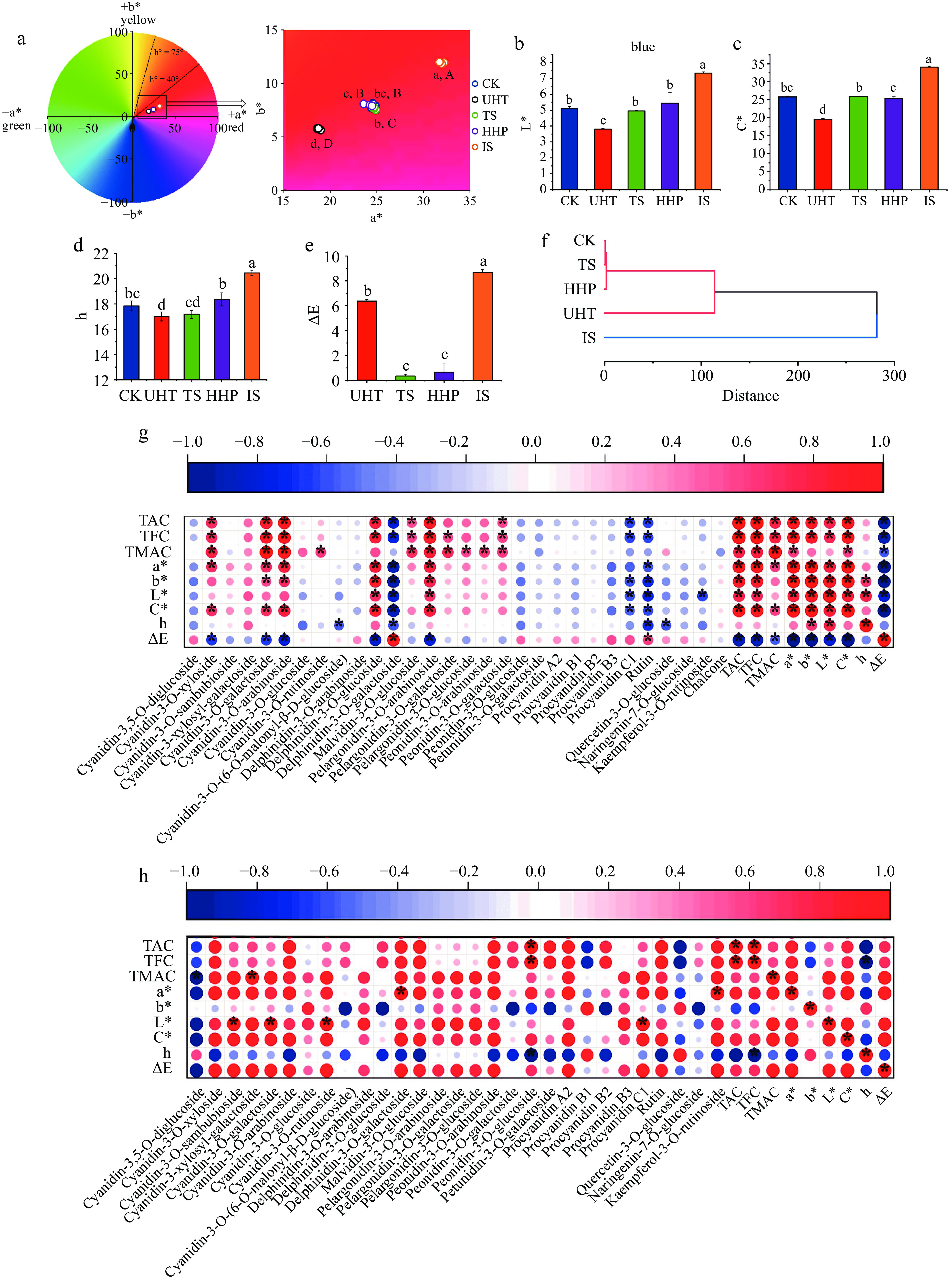
Figure 6.
The color analysis of AMJ under different sterilization methods. (a) Chromaticity distribution map; (b) L*; (c) C*; (d) h; (e) ΔE; (f) HCA analysis; (g) Heatmap of correlation between color indexes, TAC, TFC, TMAC and each detected anthocyanin monomers of CK, UHT, TS and HHP groups; (h) Heatmap of correlation between color indexes, TAC, TFC, TMAC and each detected anthocyanin monomers of IS group. Different lowercase letters express significant differences (p < 0.05); different capital letters in (a) express significant differences of b* (p < 0.05); '*' expresses significant correlation (p < 0.05).
The results of the HCA for all the tested color indicators are displayed in Fig. 6f. When the squared Euclidean distance was 114, the CK, TS, HHP, and UHT groups were grouped together and the IS group was grouped separately. It was not until the squared Euclidean distance reached 282 that all groups were clustered into one group. In addition, considering that the coloration mechanism of the IS group may be different from that of the other four groups owing to the influence of other non-anthocyanin color substances, the samples of the CK, TS, HHP, and UHT groups were divided into one category, and the IS was grouped separately. The correlations between the color indicators and TAC, TFC, TMAC, and all detected anthocyanin monomers were analyzed, as shown in Fig. 6g & h.
In Fig. 6g, both TAC and TFC were significantly positively correlated with a* and C* (R > 0.7, p < 0.05), showing that anthocyanins and flavonoids were the major substances affecting AMJ coloration. On anthocyanin monomers, after screening out the anthocyanin monomers that hardly had a great impact on AMJ color due to their low contents (< 0.15 mg/L), a* and C* values had a significant positive correlation with C-3-O-ara (Ra* = 0.82, RC* = 0.82), P-3-O-ara (Ra* = 0.81, RC* = 0.81) and C-3-O-gal (Ra* = 0.72, RC* = 0.71), while C-3-O-xyl (R = 0.70) was only significantly positively correlated with a* (p < 0.05). In Fig. 6h, TFC showed a notable negative correlation with h (R = −1.00, p < 0.05); that is, the higher the flavonoid content, the smaller the hue angle, and the juice color changed in a redder direction. In terms of anthocyanin monomers, the a* value was mainly positively correlated with Delphinidin-3-O-galactoside (R = 1.00) and K-3-O-rut (R = 1.00) (p < 0.05), however, the contents of these two monomers (0.14 mg/L and 0.24 mg/L) were too low to have a remarkable impact on the juice color.
Therefore, C-3-O-gal, C-3-O-ara, C-3-O-xyl and P-3-O-ara were the anthocyanins mainly associated with the coloration of AMJ. Sidor et al.[50] also mentioned that the dark blue-colored chokeberries were a source of C-3-O-glu、C-3-O-gal、C-3-O-xyl, C-3-O-ara, P-3-O-gal and P-3-O-ara, which was very similar to the results of this study.
-
This study is the first to investigate the influences of four representative sterilization techniques (UHT, TS, HHP, and IS) on the physicochemical properties, functional components, antioxidant activity, and color of AMJ. Anthocyanin-targeted metabolomics was used to explore the influences of different sterilization methods on AMJ anthocyanin profiles. Correlations among AMJ antioxidant activity, color features, and functional substances were analyzed. The viscosity of AMJ was significantly reduced after sterilization, and UHT, TS, and HHP treatments significantly increased the RTT of AMJ and improved the taste of the juice. Except for HHP, the other treatments aggravated AMJ browning (p < 0.05). Both TS and HHP treatments significantly enhanced or retained TPC, TAC, and TFC in AMJ, whereas the UHT and IS treatments were detrimental to the retention of these functional substances. TS treatment was the most beneficial for the retention and enhancement of antioxidant activity in AMJ, followed by HHP, whereas IS treatment led to a remarkable decline in the antioxidant capacity and was not conducive to the retention of antioxidant activity. C-3-O-gal, C-3-O-ara, C-3-O-xyl, and C-3-O-glu were the main anthocyanin monomers present in AMJ, whereas C-3-O-gal and C-3-O-ara were the main reasons for the significant upregulation and downregulation of TMAC in AMJ after TS and IS treatment, respectively. C-3-O-sam and chalcone may be differential marker metabolites in CK, and the two anthocyanin monomers are extremely unstable in AMJ and susceptible to different sterilization methods. M-3-O-glu, Cyanidin-3-O-(6-O-malonyl-β-D-glucoside), and K-3-O-rut may be differential metabolites produced by the TS, HHP, and IS treatments, respectively. TS and HHP treatments were able retained the original color quality of AMJ to the greatest extent, and C-3-O-gal, C-3-O-ara, C-3-O-xyl, and P-3-O-ara were the main anthocyanin monomers associated with AMJ coloration. C-3-O-ara, C-3-O-xyl, and P-3-O-ara were the principal contributors to the antioxidant activity of AMJ. This study provides theoretical foundation and technical reference for the sterilization selection of AMJ, which is beneficial to the nutritional high-value treatment of Aronia melanocarpa.
-
The authors confirm contribution to the paper as follows: study conception and design: Sun X, Ma T; experimental processing, analysis and interpretation of results: Lv X, Lan T, Wang S, Li X, Bao S; draft manuscript preparation, data collection and validation: Lv X; language editing, manuscript revision: Lan T. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This study was supported by the National key research and development program (2023YFD2100304), and the Innovation Capacity Support Plan of Shaanxi Province (2024QCY-KXJ-087, 2023KXJ-171, 2023-YBNY-176).
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 Bactericidal effects of different sterilization treatments on AMJ.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Lv X, Lan T, Wang S, Li X, Bao S, et al. 2024. Comparative study on the physicochemical properties, functional components, color and anthocyanins profile of Aronia melanocarpa juice using different sterilization methods. Food Innovation and Advances 3(2): 64−74 doi: 10.48130/fia-0024-0008
Comparative study on the physicochemical properties, functional components, color and anthocyanins profile of Aronia melanocarpa juice using different sterilization methods
- Received: 18 January 2024
- Revised: 22 March 2024
- Accepted: 25 March 2024
- Published online: 16 April 2024
Abstract: Investigating the influences of different sterilization methods on overall juice quality is essential for the production of high-quality juice. The effects of ultra-high temperature instantaneous sterilization (UHT), thermosonication (TS), high hydrostatic pressure sterilization (HHP), and irradiation sterilization (IS) on the physicochemical properties, functional components, and color of Aronia melanocarpa juice (AMJ) were investigated. In addition, anthocyanin target metabolomics were used to explore the influences of different sterilization methods on the AMJ anthocyanin profile. All sterilization treatments effectively ensured the microbial safety of AMJ, and the AMJ viscosity was noticeably declined after sterilization (p < 0.05). Except for HHP, the other treatments aggravated AMJ browning (p < 0.05). Both TS and HHP treatments significantly enhanced or preserved the total polyphenols, flavonoids, and anthocyanins in AMJ and retained the original juice color, whereas UHT and IS treatments were not conducive to maintaining these characteristics. TS treatment significantly increased cyanidin-3-O-galactoside (C-3-O-gal) and cyanidin-3-O-arabinoside (C-3-O-ara) contents in AMJ by 7.98% and 5.90%, while IS resulted in a significant decrease of 15.74% and 10.46% (p < 0.05). C-3-O-gal and C-3-O-ara were the major reasons for the significant upregulation and downregulation of the total monomeric anthocyanins contents (TMAC) in the AMJ after TS and IS treatment, respectively. Malvidin-3-O-glucoside (M-3-O-glu), Cyanidin-3-O-(6-O-malonyl-β-D-glucoside) and Kaempferol-3-O-rutinoside (K-3-O-rut) might be markers of differential metabolites produced by the TS, HHP, and IS treatments, respectively. Correlation analysis indicated that Cyanidin-3-O-xyloside (C-3-O-xyl), C-3-O-ara, and Pelargonidin-3-O-arabinoside (P-3-O-ara) might be the principal contributed to the antioxidant capacity of AMJ. The research results are anticipated to supply technical reference for AMJ processing.













