-
There are many kinds of food-grade nutraceuticals which are derived from a wide range of sources. Some important nutraceuticals have the functions of anti-inflammatory, anti-diabetic, anti-cancerous, and improving digestion, such as resveratrol, naringin, quercetin, carotenoids, fat-soluble vitamins, and flavonoids[1]. Nutrient fortification has been carried out in some foods to improve human health. However, some food-grade nutraceuticals have undesirable flavors, such as tannins, which are strongly astringent[2]. Besides, most food-grade nutraceuticals are highly sensitive to harsh environments, and their bioaccessibility or bioavailability after oral administration is low, which makes their application greatly restricted. For example, when affected by light, heat, and different acid-base conditions during processing, food-grade nutraceuticals often decompose and lose their efficacy[3]. In addition, the high concentration of salt ions, the complex enzyme digestion system, and different kinds of microbiota in the digestive tract will also cause the loss of activity of food-grade nutraceuticals. Even if food-grade nutraceuticals can reach the intestinal tract, their bioaccessibility will be greatly reduced due to their hydrophobicity[1]. If the food-grade nutraceuticals need to enter the bloodstream to work, they must cross three sequential cellular barriers including apical endocytosis, intracellular trafficking, and basolateral exocytosis[4]. Even if the food-grade nutraceuticals enter the blood circulation, they are difficult to accumulate in the target organs and often cause damage to non-target organs.
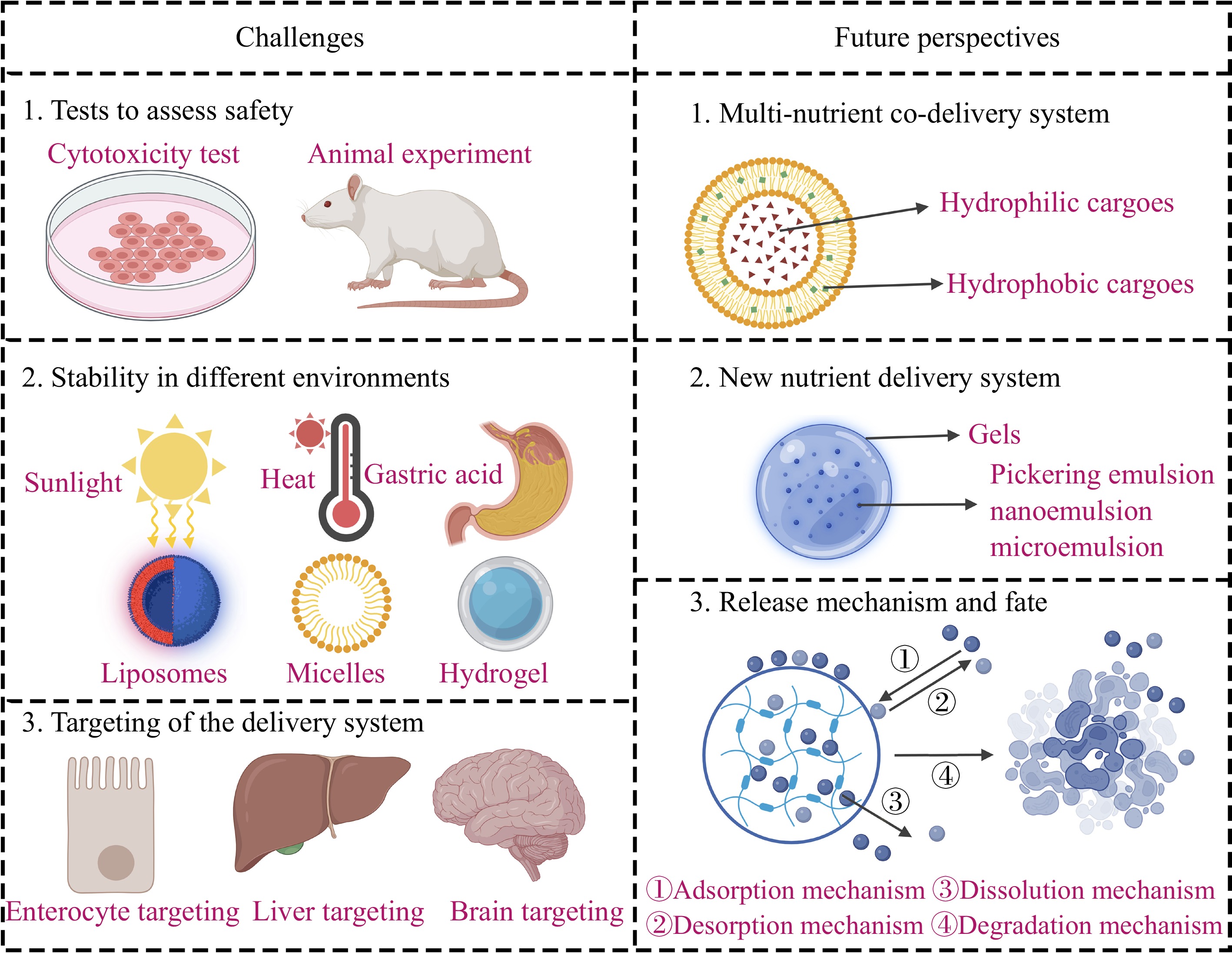
With the development of food nutrient delivery system technology (FNDS), especially promoted by the drug delivery technology, the utilization barrier of food-grade nutraceuticals has been gradually overcome, so that food-grade nutraceuticals are more and more effectively used. These FNDS can improve the solubility of food-grade nutraceuticals, enhance resistance to the environment, effectively transport food-grade nutraceuticals into the body, and target them to the organ[5]. However, the industrial application of FNDS faces many challenges. In addition, the future development direction of FNDS might affect the future development of the food industry (such as the functional beverage industry). Therefore, the challenges and future perspectives of FNDS are critically considered and summarized in this review as shown in Fig. 1, to promote the development and wide application of FNDS.
-
The safety of FNDS, especially nano-delivery systems, has received attention from many researchers. This is because the composition, dose, size, morphology, hydrophobicity, and hydrophobicity aggregation of delivery systems may affect their toxicity[6]. In addition, under the complex environments of the digestive tract, the FNDS may react with the substances in the body or in the delivery system, affecting its metabolic fate, and eventually may have side effects on the body. Another point to consider is that the nano-FNDS, due to its small size can easily penetrate biological barriers such as intestinal mucus and tight junctions, enter the bloodstream, and eventually accumulate in different organs[7]. Long-term exposure to nanocarriers may result in cytotoxicity, fibrosis, oxidative stress, immune response, inflammation, and so on[7]. Inorganic nanocarriers, such as silicon dioxide, titanium dioxide, zinc oxide, etc., are easily accumulated in the heart, liver, and kidneys, etc., and when the amount is too much, reactive oxygen species can be generated to promote oxidative stress and damage organs[8]. Food-grade biomacromolecules (such as polysaccharides, proteins, and lipids) have good biocompatibility and degradability, and can be digested in the human gastrointestinal tract[9]. Therefore, delivery systems consisting of these biomacromolecules are generally considered safe. Only in some special cases, it may be toxic to the body. Some emulsion droplets contain indigestible interfacial layers or indigestible oil phase. Therefore, these indigestible bases prevent the enzymatic hydrolysis of the droplets. These unhydrolyzed emulsion droplets can potentially be absorbed in their intact forms. As a result, their final fate is not clear and they may have potential toxic effects on the human body[10]. Besides, it might be toxic that the carbohydrates or proteins (the building block of the nutrient delivery systems) are absorbed by their intact form[9]. For example, ovalbumin can be instantly absorbed from the distal intestine via the paracellular and clathrin- and receptor-mediated endocytic pathways. Eventually, this can lead to food allergies[10,11].
Stability of food nutrient delivery systems in different environments
-
In the process of food manufacturing, transportation, storage, and marketing, FNDS is affected by environmental factors, such as temperature, humidity, light, and pH[3]. Food-grade nutraceuticals affected by these adverse environments may not only lead to the loss of precious nutraceuticals, but also greatly limit their industrial application. Additionally, the digestive environment also has a significant impact on the stability of the FNDS[1], as shown in Table 1. Although orally administered FNDS pass through the mouth very quickly, the structure of some substances may change significantly. After reaching the stomach, the FNDS may be degraded due to the highly acidic environment (pH 1.0~2.5) and the presence of various digestive enzymes, such as lipases, pepsins, and so on[12]. In the intestine, the presence of pancreatic enzymes (lipases, proteases, and amylases) and the intestinal environment with a pH of 6.0–7.0 can also cause the leakage of cargoes, so that food-grade nutraceuticals cannot reach the target organs[12]. It is worth noting that the structure of FNDS built with a single kind of protein or polysaccharide is poor in stability and is easily affected by the surrounding environment. Delivery systems are much more stable if built using two or multiple substances. For example, when whey protein and saccharide form a covalent complex, it can not only combine the advantages of the two biopolymers together, but also sometimes show new functional properties that the parent biopolymers do not have, with excellent delivery effects[13]. Bovine serum albumin (BSA) is an important building material of FNDS. Compared with BSA alone, saccharides-covalently modified BSA showed better binding strength and stability in the study of binding curcumin-carrying systems[14]. This is because the introduction of saccharides gives BSA better solubility and prevents BSA molecule aggregation. Moreover, since saccharides grafting promotes polarization of BSA molecules, the van der Waals forces and hydrogen bonds between curcumin and BSA are also enhanced[14]. In the study by Liu et al., the lactoferrin–chlorogenic acid conjugate was prepared via alkali treatment. Then, the conjugate was glycosylated with glucose by the Maillard reaction. Finally, the ternary conjugates (lactoferrin-chlorogenic acid-glucose) was obtained in this experiment. The ternary conjugates (lactoferrin-chlorogenic acid-glucose) were used to encapsulate β-carotene as a model biologically active macromolecule, and the ternary conjugates could enhance the physicochemical stability of β-carotene emulsions[15].
Table 1. Environmental conditions and functions of various parts of the human digestive tract[1].
Digestive organs Residence time pH Enzyme Ionic concentration Function Mouth cavity 
5~60 s 6.2~7.6 α-amylase 0.060 M Chewing breaks down food components and saliva acts as the lubricant. Amylase can catalyze the hydrolysis of starch into maltose, glucose, dextrin and so on. Stomach 
30 min~4 h 1.0~2.5 Pepsin, lipase 0.152 M The stomach mainly produces protein enzymatic hydrolysis, lipase hydrolysis and other reactions, and the extremely low pH can effectively kill microorganisms. Small intestine 
1~2 h 6.0~7.4 Lipase, pancreatin 0168~0.172 M The absorption of nutrients mainly occurs in the small intestine, including the release of endogenous active ingredients and the breakdown of food by endogenous enzymes. Colon 
12~24 h 5~7 Microbiota-secreted enzyme 0.100 M The colon provides a natural environment for the growth of microbial flora, and nutrients in the colon mainly interacts with the colonized microbial flora. Targetability of food nutrient delivery systems
-
In addition to protecting nutrients from destruction, another very important function of FNDS is to deliver cargo to target organs. However, in the process of transportation, due to the changes in the surrounding environment, the delivery system is easily damaged and it is difficult to reach the target location, as described previously. The mechanism of targeting mainly depends on the ability of FNDS to respond to changes in the microenvironment of the digestive tract. At present, some FNDS such as pH response, enzyme response, mucus penetration, and adhesion have been reported[16]. Generally, pH-sensitive FNDS contain groups that are sensitive to hydrogen ions and hydroxide, which can trigger force changes between intramolecular or intermolecular with pH changes, and then show changes in the properties of FNDS at the macro level. For example, the pH-sensitive hydrogels contain weakly acidic or weakly alkaline groups. Changes in pH can cause changes in the equilibrium state between the ionized and non-ionized types of these groups[17]. At low pH, the protonated acidic group interacts with the electronegative group in the hydrogels, and the hydrogel's pore size decreases and they are shrinking on a macro level. The nutraceuticals are not released at this time. With the increase of pH, the acidic groups dissociate, and the mutual attraction between polymer molecules weakens, resulting in the expansion of hydrogel pore size. Then, the cargoes will be released[18]. The FNDS responding to enzymes in the colon must be degraded and released by the catalysis of biological enzymes such as β-mannanase and glucanase, which are secreted by colon bacteria. The mucus penetration delivery system needs to be small and able to move through the mucus without restriction. For example, in the study of Li et al., they found that the pea albumin isolates hydrolyzed by enzymes trypsin was successfully used to prepare pea protein nanomicelles with gastrointestinal stability and strong permeability. Using capsaicin as a hydrophobic nutrient model, the nanocarrier system can effectively penetrate the mucus, increase the permeability of capsaicin by 2.5 times, and has excellent ability to overcome the mucus barrier[19].
-
With the improvement of living standards, human lifestyle is also changing. Single nutrients in daily life can no longer meet the growing health needs of human beings. Therefore, people pay more attention to the diversity and balance of nutrient intake. Developing the FNDS loaded with multiple nutraceuticals, that is, a multi-nutrient co-delivery system, is expected to become the future development direction. It has been reported that the multi-nutrient co-delivery system possesses several advantages such as achieving synergistic effects, formulating the proportion and dosage of different food-grade nutraceuticals, and controlling the release of food-grade nutraceuticals[1]. For example, liposomes are often used to co-encapsulate hydrophobic and hydrophilic substances because they have both a lipid bilayer and an aqueous compartments[1]. In the study by Krishna et al.[20], a droplet-based microfluidic device was used to synthesize the curcumin and quercetin co-encapsulated liposomes (made of phosphatidylcholine and cholesterol). The greatest encapsulation achieved for quercetin and curcumin was 36% ± 2.7% and 68% ± 9.2%, respectively. In vitro studies on FaDu oral carcinoma cells revealed that the co-delivery of quercetin and curcumin within liposomes synergistically enhanced their anticancer properties compared to liposomes with either quercetin or curcumin[20]. In addition, nanoparticles, emulsions, microcapsules, hydrogels, and related products have also been studied for nutraceutical co-delivery[21].
Designing new food nutrient delivery systems
-
Most of the FNDS studied by previous researchers contain only one type of carrier, such as liposomes, emulsions, gels, etc. If two or more FNDS with different advantages are combined to develop a compound FNDS, they will have the advantages of different FNDS at the same time and may have a synergistic effect to achieve more efficient nutrient delivery. For example, emulsion gels, also known as gelled emulsions or emulgels, are complex colloidal materials in which both emulsion droplets and gels exist[22]. The gel materials of emulsion gels are usually polysaccharide (e.g., κ-carrageenan, alginate, and starch), and protein (e.g., gelatin and WPI), which can resist the destruction of the emulsion by stomach acid and protease. Due to mechanical action, chemical reaction, enzyme catalysis, and other processes, the emulsion will be released from the gel matrix, incorporated in the co-digested lipid droplets, interact with endogenous lipid surface-active compounds (mainly phospholipids and bile salts) promoting the formation of mixed micelles, and eventual transportation of the mixed micelles to the small intestinal epithelium[22]. In the study by Zhao et al., model hydrophilic (riboflavin) and hydrophobic (lycopene) ingredients were encapsulated in the internal water and oil phases of water-in-oleic acid in-water (W/O/W) emulsions, respectively. These emulsions were then dispersed into calcium alginate gels. Finally, it was found that the delivery system was able to stabilize in the simulated gastric fluid and release nutraceuticals in the simulated intestinal fluid. It not only improved the photostability of nutraceuticals but also improved the bioaccessibility of co-delivered nutraceuticals[23].
Exploring the release mechanism and final fate of food-grade nutraceuticals
-
At present, most of the research on the release mechanism of FNDS has been carried out in vitro simulation conditions, rather than in animals or even humans[24]. A thorough understanding of the release mechanism of nutraceuticals and their final fate in the body will not only help to better design FNDS with more complete functions but also expand their application in the food industry. The common release mechanisms include desorption mechanisms (adsorption and desorption of food-grade nutraceuticals and FNDS), diffusion mechanisms (diffusion of nutraceuticals through the carrier matrix or capsule wall), dissolution mechanisms (dissolution of nutraceuticals in the release medium), and degradation mechanisms (release of nutraceuticals after the degradation of carrier materials, including swelling and corrosion of polymer materials) (Fig. 1)[25,26]. The release of nutraceuticals from FNDS mainly depends on the interaction of nutraceuticals, carrier, and release medium. If the binding force between the nutrient and the carrier is greater than the force between the carrier and the releasing medium, the nutrient release is mainly based on the degradation mechanism, and the release rate is mainly controlled by the swelling and dissolution process of the carrier material[27]. On the contrary, if the nutrient release mechanism is mainly diffusion, the release rate is controlled by the nutrient migration and diffusion process. When the nutrient is uploaded to the carrier in the form of adsorption, the drug is released through the process of desorption. After the nutraceuticals arrive in the intestine intact and are successfully released, it is still unclear whether they can enter the bloodstream to reach the targeted organs[26]. There are four ways for carriers and nutraceuticals to pass through the intestinal mucosal barrier. First, hydrophobic carriers mainly pass through the intestinal mucosa through the transcellular pathway; second, hydrophilic carriers cannot pass through the cell membrane and must first be transported through the cell bypass (but are limited by tight junction). The third is receptor-mediated nutrient uptake through intestinal mucosal channels. The fourth is the efflux pump on the intestinal mucosa to expel nutraceuticals through the cell membrane[28]. Li et al. found that nanotubes loaded with mangiferin could be prepared by self-assembly of hydrolyzed α-lactalbumin peptide fragments. The nanotubes were found to instantly and reversibly open the tight junctions between cells, facilitating the transport of nutraceuticals into the bloodstream[29].
-
FNDS can improve the solubility of food-grade nutraceuticals, enhance resistance to the environment, effectively transport food-grade nutraceuticals into the body and target specific organs. However, the current safety assessment of FNDS is not enough, and more extensive toxicological tests should be conducted in the future to ensure the safe use of FNDS. To protect food-grade nutraceuticals from destruction, developing FNDS that can resist harsh environments remains a thorny problem. An important function of FNDS is targeted delivery. However, the current oral FNDS have poor targeting in the body, especially to achieve targeting of internal organs or specific cells. In the future, the development direction of FNDS might be to develop multi-nutrient co-delivery systems to play the synergistic effect of food-grade nutraceuticals. Designing novel FNDS can combine the advantages of different delivery systems, such as the stability of the gel and the ability of liposomes to simultaneously load hydrophilic and hydrophobic food-grade nutraceuticals. In addition, attention should also be paid to the mechanism by which nutraceuticals are released from FNDS and the final fate of nutraceuticals so that the nutraceuticals can fully exert their effects. There is no doubt that the industrial application of FNDS is still a long way off, and there is still the need for further exploration in the future.
-
The authors confirm contribution to the paper as follows: writing - original draft, writing - review and editing, funding acquisition: Zhang Y; conceptualization: Wang Y, Xue Y; software: Wang Y, Zhang K; investigation: Zhang K; visualization: Lin X; formal analysis: Xue Y; validation: Zhang Z; methodology: Lin X, Zhang Z. All authors reviewed the results and approved the final version of the manuscript.
-
Data supporting this work is available within the article.
This study is supported by the Chinese Universities Scientific Fund (2024TC177).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang Y, Wang Y, Zhang K, Lin X, Xue Y, et al. 2024. Out of the box thinking: challenges and future perspectives for food-grade nutraceutical delivery systems. Food Materials Research 4: e027 doi: 10.48130/fmr-0024-0022
Out of the box thinking: challenges and future perspectives for food-grade nutraceutical delivery systems
- Received: 29 August 2024
- Revised: 02 October 2024
- Accepted: 09 October 2024
- Published online: 29 October 2024
Abstract: Food-grade nutraceuticals often have unstable properties and are easily decomposed under the influence of light, heat, pH, and other conditions during processing and digestion. Moreover, many hydrophobic nutraceuticals are characterized by poor solubility, low bioaccessibility, and low bioavailability, which limits the widespread utilization of food-grade nutraceuticals. With the development of food-grade nutraceutical delivery system technology (FNDS), the utilization barrier of food-grade nutraceuticals has been gradually overcome, so that food-grade nutraceuticals are increasing in their effective used. However, the development of FNDS still faces many challenges. Herein, the safety of FNDS is first discussed. In addition, the stability of FNDS in different environments remains to be improved. Besides, the FNDS also has the challenge of off-target effect. In the future, the development direction of FNDS might be exploring the multi-nutrient co-delivery system, designing new types of FNDS, and clarifying the nutraceuticals release mechanism, and their final fate. The challenges and future perspectives of FNDS have been considered critically and summarized in this review, to promote the development of FNDS and the wide application of nutraceuticals.
-
Key words:
- Food-grade nutraceuticals /
- Delivery systems /
- Target /
- Stability /
- Release mechanism