-
Tea, a beverage with a long history and widely loved by consumers contains many active substances such as tea polyphenols, catechins, alkaloids, tea polysaccharides, etc., and these ingredients have a variety of health benefits, such as antioxidant, hypoglycemic, hypotensive, hypolipidemic, and antibacterial effects[1−4]. Green tea is the largest tea variety produced in China, which is classified as an unfermented tea with high nutritional value. From fresh leaves to the final product, green tea goes through multiple steps such as fixation, rolling, and drying[5]. Fixation (tea killing green) is a crucial step in tea processing, which involves heating the freshly picked tea leaves to terminate further enzymatic oxidation, preserve the color, aroma, and flavor of the tea, and prepare them for subsequent processing steps. Depending on different fixation and drying methods, green tea can be further divided into pan-fired, sun-dried, baked, and steamed green tea.
Selenium (Se) has excellent antioxidant properties that can avoid the aging process caused by the oxidation of cells due to free radicals. Besides, selenium also has multiple effects, including inhibiting the growth and division of cancer cells, optimizing cytokines in serum, maintaining the stability of cell DNA, and enhancing cellular immune response[6]. Selenium has the characteristics of counteracting heavy metals such as lead, cadmium, mercury, arsenic, etc, which can generate insoluble complexes, thereby reducing the accumulation of these heavy metals in the organism[7]. Tea is a plant with selenium accumulation ability. Se-enriched teas combine the flavor and health benefits of tea with the additional wellness advantages of selenium, holding promising market potential[6,8]. Meanwhile, the application of exogenous selenium fertilizer can increase tea yield, improve tea quality, and enhance the tea plant's resistance to various biotic and abiotic stresses, including pests and diseases, as well as exogenous hazardous substances (such as pesticides and heavy metals)[9,10]. According to the standards of the Chinese Ministry of Agriculture (NY/T 600-2002), Se-enriched teas refer to the buds, leaves, and tender stems of tea tree shoots grown in selenium-rich soil. After specific processing, the selenium content of Se-enriched teas should be controlled within the range of 0.25−4.00 mg/kg. Selenium-rich green tea showed more protective effects against lipid peroxidation and free radical scavenging ability than ordinary green tea[11]. Compared with conventional tea, the aqueous extract of selenium-rich green tea showed superior inhibitory effects on HepG-2 cells[12]. Selenium-rich green tea has a significant protective effect on liver fibrosis triggered by carbon tetrachloride (CCl4), and further screens high-content antioxidant substances from rat liver tissue, serum, and urine samples[13]. Selenium polysaccharide components from Se-enriched teas were confirmed to be α-galacturonic acid transferase inhibitors, peroxidase activity inhibitors, and superoxide dismutase activity enhancers[14]. Therefore, Se-enriched teas are attracting favorable attention from researchers and consumers as a promising natural source of Se supplementation.
In the processing of tea infusion, brewing is a crucial step to determine the quality of the tea infusion. Proper brewing conditions before the processing of tea extract can effectively improve the quality of tea extract. The influencing factors that determine the quality of tea infusion include types and appearance of tea, brewing temperatures, water/tea ratio, brewing duration, brewing times, and water quality[5]. The brewing temperature is pivotal in determining the quality of the tea infusion. Based on diffusion principles, the dissolution speed of tea polyphenols and caffeine accelerates as the brewing temperature rises[15]. Moreover, extending the brewing duration can inhibit the precipitation of tea extract. Therefore, it is recommended to appropriately extend the brewing duration to ensure saturated and uniform dissolution, ultimately achieving better extraction of tea pigments like theaflavins (TFs), thearubigins (TRs), and theabrownins (TBs)[16]. While high brewing temperatures may increase the dissolved amount, improper thermal treatments can lead to the oxidation and browning of tea active components with a bitter and turbid taste[17]. Conversely, the low brewing temperature may reduce the dissolution efficiency and weaken the flavor of tea infusions[18]. Although prolonging the brewing duration allows for a higher dissolution rate of the main substances of tea, long brewing duration may cause adverse effects, including browning of the tea infusion color, increased turbidity, and the loss of aroma[19,20].
The quality of tea infusion is paramount for tea processing and consumption. To the best of our knowledge, there has been a lack of systematic studies examining the dynamic changes of quality components in Se-enriched green teas under various brewing conditions (temperature, duration, and time). Therefore, this study focuses on Se-BF and Se-YL to elucidate their chemical profiles, dissolution patterns, sensory attributes, and in vitro biological activities, providing a scientific foundation for rational tea consumption and the development of industrial tea beverages.
-
Three different batches of green tea samples were produced in 2021, 2022, and 2023, generously provided by Enshi Selenium Impression Agricultural Technology Co., Ltd (Hubei Province, China). The ordinary Xiazhou Bifeng and Enshi Yulu green teas were termed BF and YL, respectively, while the Se-enriched Xiazhou Bifeng and Enshi Yulu green teas were named Se-BF and Se-YL, respectively. Specific information about the tea samples can be found in Supplementary Table S1. All teas were packaged properly in sealed bags and stored in a dry environment. For long-term storage, teas were refrigerated at −20 °C. Glucosamine selenium (GlcN-Se) was synthesized by Jiangsu Shuanglin Marine Biology Group Co., Ltd. (Jiangsu, China) with selenium content of 4.00% ± 0.20%. All other chemical agents were of analytical grade and provided by Sinopharm Chemical Reagent Co., Ltd. DPPH and ABTS were provided by Aladdin Industrial Corporation (Shanghai, China). α-glucosidase and α-amylase were purchased from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China).
Chemical profiles
-
The soluble protein content was determined by the Coomassie brilliant blue method according to the description of the National Standard of China (SN/T 3926-2014). Tea polysaccharide content was determined by the phenol sulfuric acid method according to the National Standard of China (SN/T 4260-2015). The content of total flavonoids was measured by the spectrophotometer method by the National Standard of China (SN/T4592-2016). The theanine content was determined according to the National Standard of China (GB/T8314-2013). The contents of catechins and caffeine were determined using an LC-20A high-performance liquid chromatography (HPLC) system (Shimadzu, Tokyo, Japan). The spectrophotometric method was adopted to determine the total polyphenol content in tea in accordance with the National Standard of China (GB/T 8313-2018). The content of minerals was determined by Optima 8000 inductively coupled plasma emission spectrometer (Perkin Elmer, MA, USA). The content of tea pigments (theaflavins, thearubigins, and theabrownins) were determined using a T6PC UV Spectrophotometer (Beijing Puxi general instrument) according to previous studies with some modifications[21−23]. Concerning the agricultural industry standard in China (NYT 3082-2017), the spectrophotometric method is adopted to determine the chlorophyll content with some modifications. Concerning the National Standard of China (GB 5009.268-2016), the ICP-MS method is adopted to determine the selenium content of teas.
Preparation of tea infusion and dissolution pattern of tea active ingredients
-
One gram of tea leaves was weighed into a beaker with the addition of 50 mL of water and was immediately placed in a water bath at 65, 75, 85, and 100 °C for 1, 2, 3, 4, 5, 10, 20, and 40 min. After brewing, the tea infusion was filtered and kept for the determination of the dissolution rates of tea active ingredients. The content of different tea active ingredients measured under the conditions of 100 °C and 40 min is taken as 100% of dissolution rate to calculate the dissolution rates of tea active ingredients in different teas under various brewing conditions.
Sensory evaluation of tea infusion
-
A sensory evaluation team composed of 30 well-trained sensory evaluation panelists recruited from Shanghai Normal University conducted a sensory evaluation on tea infusions concerning GB/T 23776-2018 'Tea Sensory Evaluation Method'. The team members were aged between 20 and 30, with equal numbers of males and females. Each panelist had at least 1 year of experience in sensory evaluation of tea and has completed the basic smell test, aroma matching test, aroma ranking test, sensory description ability test. Before the experiment, the study design and procedures were thoroughly reviewed and ethically approved by the ethics committee. The rights and privacy of all participants were utilized during the execution of the research. The participants have given their consent to take part in the sensory study and use their information. To ensure fairness, the tea samples were coded and randomly placed, and an anonymous evaluation method was adopted. The sensory evaluation was conducted from five aspects: leaf residue, aroma, color, taste, and clarity. The scoring criteria are listed in Supplementary Table S2. After tasting each sample, the team members rinsed their mouths to avoid intervention between samples. The entire process required careful and objective judgment to ensure the accuracy and reliability of the results.
Establishment of a daily tea drinking model
-
Based on the preliminary research findings of this study, combined with consumers' daily tea-drinking habits and the tea evaluation methods stipulated in Chinese national standards, the following brewing conditions were selected to establish a daily tea-drinking model to explore the impact of brewing times on the extraction of tea active ingredients and their in vitro bioactivities: brewing temperature of 100 °C, tea-to-water ratio of 1:50, with a total of four brewings. Specifically, the brewing time for the first, second, and third infusions was 5 min each, while the brewing time for the fourth infusion was designed to be 1 h to ensure sufficient leaching of tea active ingredients.
Antioxidant activity assay
DPPH radical scavenging activity
-
The DPPH radical scavenging ability of all tea infusions was measured[24]. In brief, a 0.1 mM ethanol solution (2 mL) of DPPH radicals was added to a water solution (1 mL) of tea infusion at 0, 0.4, 0.8, 1.2, 1.6, and 2.0 mg/mL, respectively. The absorbance of the resulting mixture was measured against a blank sample at 517 nm after 30 min in the dark at room temperature. The DPPH radical scavenging activity was calculated according to the following Eqn (1):
$ DPPH\;radical\;scavenging\;rate\;\left({\text{%}}\right)=\left[\left(\dfrac{{{A}_{0}-A}_{i}}{{A}_{0}}\right)\right]*100 $ (1) where, Ai is the absorbance obtained from a sample and A0 is the absorbance of the control. Results were expressed as the percentage of inhibition of DPPH radical scavenging.
Total antioxidant capacity assay
-
The total antioxidant capacity of these tea infusions was quantified by the total antioxidant capacity assay kit (ABTS method)[24]. Briefly, 10 μL of the water solution of samples at 2.0 mg/mL were mixed with 20 μL of peroxidase solution and 170 μL ABTS solution. The reaction mixtures were reacted in the dark at room temperature for 6 min using a microplate reader (Powerwave XS, Biotek, USA). The MTrolox standards at 0.1, 0.2, 0.4, 0.8, and 1.0 mM, and the blank were prepared in the same manner as the samples. The absorbance of the reaction mixture was measured at 734 nm. The total antioxidant capacity was expressed as Trolox equivalent after blank subtraction.
α-amylase and α-glucosidase inhibition assay
-
The determination of α-amylase and α-glucosidase enzyme inhibition assay followed a previously described method with some modifications[25]. Briefly, for the α-amylase assay, 500 μL of the tea infusions with different concentrations, or positive control (1 mM acarbose) was added to 500 μL of 13 U/mL α-amylase solution (type VI-B from porcine pancreas in 0.02 M sodium phosphate buffer pH 6.9) and incubated in test tubes at 25 °C for 10 min before 500 μL of 1% soluble starch solution (previously dissolved in sodium phosphate buffer and boiled for 15 min) was added to each tube and incubated for another 25 min. Finally, 1 mL of dinitrosalicylic acid color reagent was added and the tubes were placed in 100 °C water baths for 5 min. The mixture was diluted with 100 mL of distilled water. The absorbance was read at 520 nm. Results were presented as percent inhibition according to Eqn (2).
$ \alpha {\text -}Amylase\;inhibition\;rate\;\left({\text{%}}\right)=\dfrac{{A}_{control}-\left({A}_{test}-{A}_{blank}\right)}{{A}_{control}}\times 100{\text{%}} $ (2) where, Acontrol is the absorbance of sample without tea infusions, Atest is the absorbance of sample containing tea infusions, and Ablank is the absorbance of sample containing tea infusions, but without enzyme solution.
For the α-glucosidase assay, in a 96-well plate, 50 μL of BTE with different concentrations, or positive control (1 mM acarbose) was added to 100 μL of a 1 U/mL α-glucosidase solution (in 0.1 M sodium phosphate buffer pH 6.9) and incubated for 10 min. A 50 μL aliquot of a 5 mM p-nitrophenyl-α-d-glucopyranoside (PNPG) solution (in 0.1 M sodium phosphate buffer pH 6.9) was added briefly to each well and incubated at 25 °C for 5 min before the absorbance was read at 405 nm. Results were presented as percent inhibition according to Eqn (3).
$ \alpha {\text-}Glucosidase\;inhibition\;rate\;\left({\text{%}}\right)=\dfrac{{A}_{control}-\left({A}_{test}-{A}_{blank}\right)}{{A}_{control}} \times 100{\text{%}} $ (3) where, Acontrol is the absorbance of sample without tea infusions, Atest is the absorbance of sample containing tea infusions, and Ablank is the absorbance of sample containing tea infusions, but without enzyme solution.
Statistical analysis
-
All experiments were performed in triplicate and the reported results were the averages of experiments. The data were subjected to statistical analysis of variance using Origin 2021 (OriginLab Corporation, Northampton, MA, USA) and SPSS 18.0 for Windows (SPSS Inc., Chicago, USA), including quadratic polynomial stepwise regression analysis. Statistical differences were determined by one-way analysis of variance (ANOVA) with Duncan's post hoc test and the least significant differences (p < 0.05) were accepted among the treatments.
-
The quality of tea is intimately linked to its chemical components. Supplementary Table S3 presents the contents of caffeine, soluble sugar, soluble protein, total free amino acids, flavonoids, and polyphenols in different teas. Tea polyphenols and caffeine contribute to the bitter taste of tea infusions, whereas soluble sugar and free amino acids help mitigate the bitter taste and enhance the freshness of tea infusions. Notably, there were significant differences in the chemical compositions among different teas (p < 0.05). The caffeine content varied from 24.06 to 43.24 mg/g, surpassing the minimum standard of 24 mg/g for teas. The total soluble sugar content ranged from 42.44 to 66.10 mg/g, while the soluble protein content spanned from 7.28 to 10.79 mg/g. The content of free amino acids fell within the range of 58.8 to 75.4 mg/g, and the tea polyphenol content ranged from 124.35 to 164.84 mg/g. Furthermore, comparisons between four different batches of tea revealed significant differences in their contents (p < 0.05), suggesting that influencing factors such as the growth environment of tea trees may contribute to variations in chemical compositions.
The average contents of main chemical components in BF, Se-BF, YL, and Se-YL are shown in Fig. 1a. The contents of caffeine and polyphenols in BF and YL were both higher than those in the corresponding selenium-enriched teas, which was consistent with our previous research, indicating that the application of selenium fertilizer to tea reduced the contents of polyphenols and caffeine[10]. However, no obvious effect of exogenous selenium fertilizer was observed on soluble protein, soluble sugar, free amino acids, and flavonoids. Interestingly, YL exhibited higher contents of caffeine, soluble total sugar, and soluble protein compared to BF, potentially attributable to differences in processing techniques, which could alter the chemical compositions of teas, ultimately impacting its quality[26]. YL employs steaming as its fixation technology, which aids in preserving original chemical components, whereas BF utilizes high-temperature roasting, leading to the loss of these components. Steaming technology is more effective in retaining higher soluble sugar content and total free amino acid content compared to roasted green tea[27].
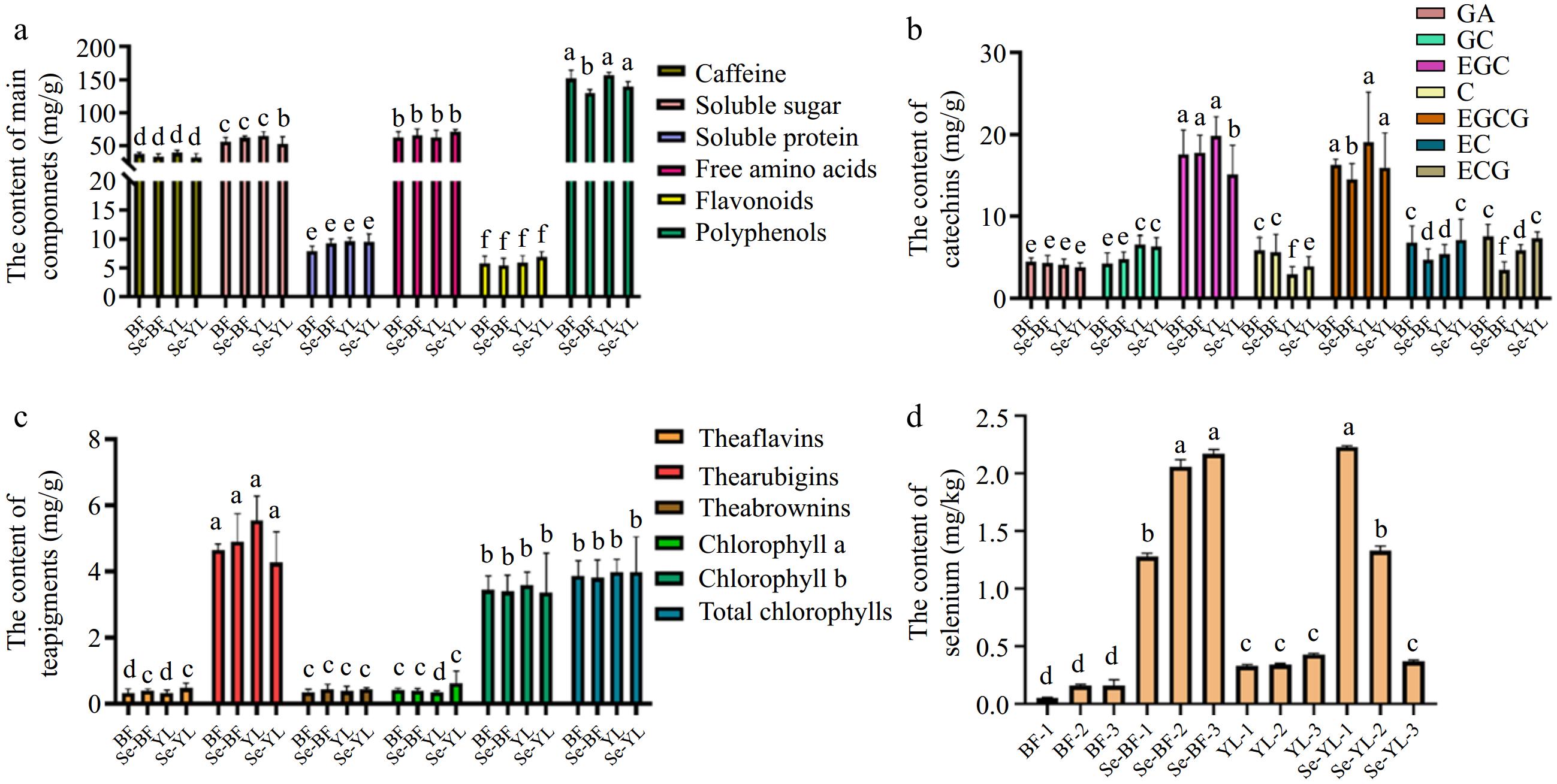
Figure 1.
The average content of (a) main chemical components, (b) catechins, (c) tea pigments, and (d) selenium of four kinds of tea (different superscripts represent significant differences between different teas, p < 0.05).
The content of catechins in tea
-
The catechin contents of 12 tea samples are listed in Supplementary Table S4. Catechin is the most important phenolic component in tea containing a variety of monomers. The catechin contents in selenium-enriched green teas are mainly EGCG and EGC, and EGCG is considered the most important antioxidant in green teas, accounting for about 30% of the total antioxidant capacity[26]. There were significant differences in catechin contents among different teas (p < 0.05). The GA content is between 3.10−5.21 mg/g, GC content is between 2.74−7.86 mg/g, EGC content is between 11.34−21.34 mg/g, +C content is between 1.99−7.37 mg/g, EGCG content is between 10.05−22.98 mg/g, EC content is between 3.05−10.06 mg/g, and ECG content is between 2.21−8.99 mg/g. The average contents of catechins in BF, Se-BF, YL, and Se-YL are shown in Fig. 1b. The contents of GA and GC in BF and YL were higher than those in their corresponding selenium-enriched green teas, while no effect of exogenous selenium fertilizer was found on other catechins.
Content of tea pigments
-
As shown in Supplementary Table S5, this study explored the effects of different natural pigments on the quality of tea. Chlorophyll, an important tea pigment, affects the appearance and leaf color of tea. The tea polyphenols will undergo oxidation to form theaflavins (TFs), which are further oxidized to yield thearubigins (TRs). Thearubigins may combine with other substances to form theabrownins (TB), which not only affect the color of tea infusion but also increase the mellow taste of tea infusion[28]. There were significant differences in the content of tea pigments among teas (p < 0.05). The concentration of theaflavins ranged from 0.22 to 0.65 mg/g; the concentration of thearubigins ranged from 3.21 to 6.39 mg/g; the concentration of theabrownins ranged from 0.24 to 0.64 mg/g and the concentration of total chlorophyll ranged from 3.08 to 5.52 mg/g. Notably, thearubigins exhibited the highest content in all tea samples, suggesting that TRs had not yet been fully converted into TBs. Moreover, substantial differences were observed between samples of the same tea type from different batches (p < 0.05), highlighting the instability of tea pigments. Figure 1c illustrates the average content of natural pigments in tea. After selenium biofortification, the contents of theaflavins, theabrownins, chlorophyll a, and chlorophyll in selenium-rich green teas were higher than those in ordinary green teas, except for chlorophyll b, indicating that the application of selenium fertilizer may affect the content of tea pigments, which was consistent with our previous study[10]. Additionally, the supplementation of selenium in growth medium or nutrient solution could elevate the net photosynthetic rate, stomatal conductance, and transpiration rate of various plants[29], which might be related to the improvement of chlorophyll content by selenium fertilizer, ultimately contributing to increased tea yield.
Content of selenium
-
As shown in Fig. 1d, the selenium content of Se-BF and Se-YL was significantly higher than that of BF and YL. The selenium content in selenium-rich tea is stipulated to be between 0.25−4.00 mg/kg (NY/T 600-2002). The selenium content in BF ranged from 0.05 to 0.16 mg/kg, while the selenium content in Se-BF ranged from 1.28 to 2.17 mg/kg, which met the standard of selenium-rich tea. Meanwhile, although no exogenous selenium fertilizer was applied to YL, its selenium content ranged from 0.33 to 0.43 mg/kg, which met the standard of selenium-rich tea. After the application of exogenous selenium, the selenium content in Se-YL reached 0.37−2.23 mg/kg. The phenomenon was that attributed to its planting area (Enshi, Hubei, China), which is currently recognized as a selenium-rich area[30]. It was worth noting that there were significant differences in selenium content between different batches of Se-BF and Se-YL. Many influencing factors can affect the selenium content in different plants due to various absorption rates of plants, which might be related to plant species, soil, pHs, microbial activity, precipitation, and other biogeochemical parameters[6].
Dissolution pattern of active ingredients in selenium-enriched green teas
Tea polyphenols
-
The dissolution pattern of tea polyphenols in Se-BF and Se-YL is shown in Fig. 2a and b, respectively. When the brewing temperature reached 100 °C, the dissolved amount of tea polyphenols was significantly higher than at other temperatures, regardless of brewing duration. Specifically, under the conditions of 100 °C and 40 min, the dissolved amount of tea polyphenols in Se-BF was 172.46 mg/100 mL, while in Se-YL it was 160.46 mg/100 mL, indicating that boiling water was more conducive to the dissolution of tea polyphenols. The dissolution pattern of tea polyphenols conformed to the general leaching rule of solute, and the dissolved amount was positively correlated with both brewing duration and temperature. Both Se-BF and Se-YL exhibited high leaching rates of tea polyphenols within the first 7 min of brewing. With the extension of brewing duration, especially after 30 min, the dissolution rate gradually slowed down. Overall, the dissolved amount of tea polyphenols increased with the rise in brewing temperature. Nevertheless, a high-temperature environment was prone to the oxidation and decomposition of tea polyphenols[31], hence, caution should be exercised when brewing tea at high temperatures for extended periods.
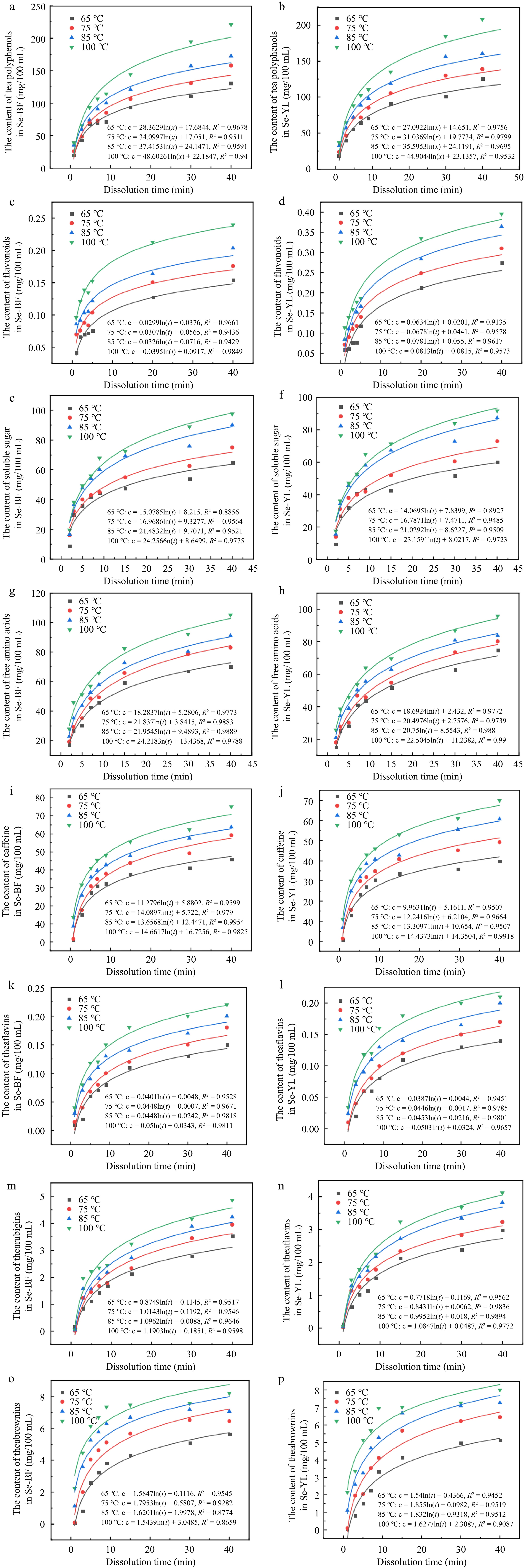
Figure 2.
Effects of different brewing temperatures and duration on (a), (b) dissolution patterns of tea polyphenols, (c), (d) flavonoids, (e), (f) soluble sugar, (g), (h) free amino acids, (i), (j) caffeine, (k), (l) theaflavins, (m), (n) thearubigins, and (o), (p) theabrownins in Se-BF and Se-YL.
As shown in Supplementary Table S6, the content of tea polyphenols measured under the conditions of 100 °C and 40 min is taken as 100% of the dissolution rate to calculate the tea polyphenols dissolution rates of Se-BF and Se-YL under different brewing temperatures and durations. Overall, the tea polyphenol dissolution rate of Se-YL was higher than that of Se-BF. When the brewing duration was 1 min, the dissolution rates of Se-YL were 9.86%, 12.91%, 17.44%, and 20% respectively, which were 1.08, 1.1, 1.09, and 1.25 times those of Se-BF. This discrepancy might be attributed to the different processing technologies of Se-BF and Se-YL. The steaming process of Se-YL may more efficiently disrupt the internal cell structure, making tea polyphenols easier to dissolve during steeping. Supplementary Table S7 presents the half dissolution time (t0.5) and full dissolution time (t1.0) of tea polyphenols under different brewing temperatures. It took 27.32, 15.20, 10.70, and 6.41 min for Se-BF to extract 50% tea polyphenols at 65, 75, 85, and 100 °C, respectively, while it took 16.93, 10.41, 6.68, and 5.14 min for Se-YL. Since tea polyphenols are the main sources of bitterness and astringency in tea infusion, the taste of tea infusion can be improved by appropriately shortening the brewing duration. Meanwhile, elevating the brewing temperature or extending the brewing duration to dissolve more tea polyphenols could enhance the health benefits of tea drinking. A quadratic polynomial stepwise regression analysis was performed on the brewing temperature (X1) and brewing duration (X2) with the concentration of tea polyphenols (Y) in Se-BF tea infusion, resulting in the regression equation Y = 0.0053X1 + 0.0139X2 + 0.0141 (R2 = 0.996). The regression equation for the brewing temperature (X1) and brewing duration (X2) with the dissolved concentration of tea polyphenols (Y) in Se-YL tea infusion is Y = 0.0063X1 + 0.0175X2 + 0.0058 (R2 = 0.995).
Flavonoids
-
The dissolution patterns of flavonoids in Se-BF and Se-YL under different brewing temperatures and durations are illustrated in Fig. 2c and d, respectively. Similar to tea polyphenols, as the brewing temperature was elevated and the brewing duration was extended, the leaching amount of flavonoids continued to rise. However, when the brewing duration exceeded 10 min, the leaching rate of flavonoids begins to decline. The dissolved amounts of Se-BF and Se-YL at 100 °C for 40 min were 0.24 and 0.39 mg/100 mL, respectively. Furthermore, the difference between the dissolved amount at 100 and 85 °C in Se-BF was greater than that in Se-YL, indicating that flavonoid compounds in Se-BF require higher brewing temperatures to be fully released.
Supplementary Table S8 shows the flavonoid dissolution rate of Se-BF and Se-YL under different brewing temperatures and durations. When the teas were brewed for 40 min at 65, 75, and 85 °C, the dissolution rates were 64.17%, 73.33%, and 84.86% of the tea infusion brewed at 100 °C, respectively, and 69.25%, 78.35%, and 92.00% of the tea infusion brewed at 100 °C, respectively. The flavonoid dissolution rate increased by 30.00%, 35.00%, 43.89%, 56.11% and 19.38%, 29.65%, 38.42%, 44.82% in the first 0−7 min at 65−100 °C, and by 34.17%, 38.33%, 40.97%, 43.89%, and 49.87%, 48.70%, 53.58%, 55.18% in the 7−40 min. This phenomenon indicated that the dissolution rate of flavonoids in Se-YL in the first 7 min was lower than that in Se-BF, which could be reversed from 7 to 40 min. The t0.5 and t1.0 of flavonoids in Se-BF and Se-YL at different brewing temperatures are shown in Supplementary Table S9. Theoretically, the brewing time required for Se-BF to dissolve 50% of flavonoids at 65, 75, 85, and 100 °C was 20.72, 10.58, 5.89, and 2.80 min, respectively, while it took 22.29, 12.96, 8.35, and 5.6 min to dissolve flavonoids in Se-YL, respectively. The quadratic polynomial stepwise regression analysis was performed on the brewing temperature (X1) and duration (X2) with the flavonoid concentration (Y) in the Se-BF tea infusion, resulting in the regression equation Y = 0.0036X1 + 0.067X2 + 0.153 (R2 = 0.9225). Similarly, the regression equation for the brewing temperature (X1) and duration (X2) with the flavonoid dissolution concentration (Y) in the Se-YL tea infusion is Y = 0.0056X1 + 0.0062X2 − 0.004 (R2 = 0.9069).
Soluble sugar
-
The soluble total sugar is the source of sweetness in the tea infusion, and the massive dissolution of soluble total sugar facilitates neutralizing of the bitterness brought by tea polyphenols. The dissolution patterns of soluble total sugar of Se-BF and Se-YL under different brewing conditions are shown in Fig. 2e and f, respectively. With the increase in the brewing temperature and duration, the leaching amount of soluble total sugar continued to rise. The dissolution quantities of Se-BF and Se-YL under the conditions of 100 °C and 40 min were 97.5 and 91.77 mg/100 mL, respectively. Meanwhile, it was observed that the dissolution pattern of Se-BF and Se-YL were similar at 85 and 100 °C, and the dissolution pattern at 65 and 75 °C were similar, indicating that soluble sugar could be dissolved in large quantities under higher brewing temperatures. Therefore, 85 °C can be selected to extract more soluble sugar when drinking tea.
Supplementary Table S10 shows the dissolution rates of soluble sugar for Se-BF and Se-YL. The dissolution rate of Se-BF and Se-YL reached the highest within 1−3 min, followed by 3−5 min and 5−7 min. After 7 min, the dissolution rate of Se-BF and Se-YL gradually decreased. Similar to tea polyphenols, the soluble sugar of Se-YL was easier to dissolve out, which might be attributed to the different processing techniques of Se-BF and Se-YL. The t0.5 and t1.0 of soluble total sugar in Se-BF and Se-YL at different brewing temperatures are shown in Supplementary Table S11. The brewing duration required for Se-BF to extract 50% of soluble sugar at 65, 75, 85, and 100 °C was 14.27, 9.56, 5.39, and 4.45 min, respectively, while the brewing duration required for Se-YL was 14.57, 9.19, 5.12, and 4.37 min, respectively. Except at low brewing temperatures, soluble total sugar in Se-YL was easier to dissolve out. The dissolution amount of soluble sugar and polyphenols should be comprehensively compared to keep them in a balanced state to enhance the sweetness of the tea infusion. In green teas, the content of polyphenols was generally three times that of soluble sugar, and the dissolution rates of both followed the principle of being fast first and then slow. With the extension of brewing duration, the dissolved amount of polyphenols exceeded soluble sugar, thus increasing the bitterness of tea infusions. The quadratic polynomial stepwise regression analysis was performed on the brewing temperature (X1) and duration (X2) and the soluble sugar concentration (Y) in Se-BF tea infusion, and the regression equation was obtained as Y = 0.3678X1 + 0.0567X2 − 0.089 (R2 = 0.9501). The regression equation for the brewing temperature (X1) and duration (X2) and the soluble sugar dissolved concentration (Y) in Se-YL tea infusion is Y = 0.4556X1 + 0.45X2 + 0.088 (R2 = 0.9017).
Free amino acids
-
Amino acids primarily contribute to the freshness of tea infusion, enhancing its inherent flavor. The dissolution patterns of free amino acids of Se-BF and Se-YL under different brewing conditions are illustrated in Fig. 2g and h, respectively. Similar to other main components, as the brewing temperature and duration increased, the leaching amount of free amino acids continued to rise. With the progress of the brewing process, free amino acids continuously dissolved from tea leaves into tea infusion, but their leaching rate gradually decreased with the extension of brewing duration, especially after 10 min. Specifically, brewed at 100 °C for 40 min, the dissolved amounts of free amino acids in Se-BF and Se-YL are 105.23 and 95.88 mg/100 mL, respectively.
Supplementary Table S12 presents the dissolution rates of free amino acids in Se-BF and Se-YL. The highest dissolution rates for both Se-BF and Se-YL occurred within the first minute, followed by the 5−7 min interval. The dissolution rates of free amino acids in Se-BF and Se-YL at 65−100 °C increased by 40.44%, 46.15%, 50.16%, 54.05%, and 42.99%, 48.89%, 52.49%, 55.97%, within 0−7 min, respectively, and by 26.29%, 32.92%, 36.42%, 45.95%, and 35.11%, 34.78%, 34.99%, 44.03% within 7−40 min, respectively. After 7 min, the leaching of both Se-BF and Se-YL was retarded, with Se-YL exhibiting a higher likelihood of extracting free amino acids, which was also influenced by its processing technology. As listed in Supplementary Table S13, the t0.5 of the free amino acids at 65, 75, 85, and 100 °C was 13.07, 8.65, 6.33, and 4.24 min for Se-BF, respectively, while the t0.5 was 10.93, 8.36, 5.87, and 4.30 min for Se-YL, further confirming that Se-YL was more likely to leach free amino acids. Through a quadratic polynomial stepwise regression analysis on the brewing temperature (X1) and duration (X2) with the concentration of free amino acids (Y) in the Se-BF tea infusion, the regression equation Y = 0.6766X1 + 0.0225X2 − 0.0139 was obtained (R2 = 0.9388). The regression equation for the brewing temperature (X1) and duration (X2) with the concentration of free amino acids (Y) in the Se-YL tea infusion was Y = 0.0178X1 + 0.3492X2 + 0.7099 (R2 = 0.9276).
Caffeine
-
Caffeine, the primary source of the bitter taste in tea infusions, serves the functions of refreshing the mind and enhancing metabolism. However, excessive consumption of caffeine often leads to addiction and can even damage the central nervous system in severe cases[32]. A continuous daily intake of 500–600 mg of caffeine (7 to 9 cups of tea) may increase potential health risks. Higher levels of caffeine intake can cause a variety of adverse effects on the health of sensitive people, including heart palpitations, gastrointestinal disorders, anxiety, hypertension, and insomnia[33]. Figure 2i and j illustrate the dissolution patterns of caffeine in Se-BF and Se-YL. The dissolution amount of caffeine was continuously elevated with the brewing temperature and duration increasing, but the dissolution rate gradually decreased with the extension of the brewing duration. When the brewing temperature was 100 °C and the brewing duration was 40 min, the dissolution amount of caffeine in Se-BF tea infusion was 75.13 mg/100 mL, while the dissolution amount of Se-YL was 60.78 mg/100 mL. At 65 °C, the dissolution amounts of caffeine in Se-BF and Se-YL were 45.71 and 39.65 mg/100 mL, respectively, indicating that the optimal brewing temperature for caffeine was above 85 °C.
As shown in Supplementary Table S14, when brewed for 40 min at 65, 75, and 85 °C, the dissolution rates of caffeine in Se-BF and Se-YL were 60.93%, 78.94%, 84.90% and 58.82%, 70.44%, 86.84% of that in tea infusion brewed at 100 °C, respectively, indicating that higher brewing temperatures facilitated the dissolution of caffeine. The dissolution rate of caffeine in Se-BF and Se-YL was the highest during the first 1−3 min, followed by the 3−5 min interval. The dissolution rate of caffeine in Se-BF and Se-YL under 65−100 °C was increased by 36.23%, 41.21%, 47.93%, 54.39%, and 33.18%, 42.80%, 45.73%, 51.43% within 0−5 min, respectively, and by 23.64%, 37.73%, 41.11%, 48.57%, and 18.31%, 27.64%, 36.97%, 38.57%, 45.61% within 5−40 min, respectively. Overall, the dissolution rate of caffeine in Se-BF was higher than that in Se-YL. According to Supplementary Table S15, the t0.5 for Se-BF to extract caffeine at 65, 75, 85, and 100 °C were 16.76, 9.91, 6.65, and 4.47 min, respectively, while the t0.5 for Se-YL were 19.98, 10.82, 6.58, and 4.51 min, respectively. This phenomenon showed that caffeine was more easily extracted from Se-BF than from Se-YL. Meanwhile, for consumers needing to stay awake, brewing tea at high temperatures for a longer duration is recommended. Otherwise, it should control the brewing duration within t0.5 or choose a lower brewing temperature, such as 75 °C. A quadratic polynomial stepwise regression analysis was conducted on the brewing temperature (X1), duration (X2), and the concentration of caffeine (Y) in Se-BF tea infusion. The regression equation obtained is Y = 0.00037X1 + 0.0256X2 + 0.0089 (R2 = 0.9562). For the brewing temperature (X1), duration (X2), and the caffeine concentration (Y) in Se-YL tea infusion, the regression equation is Y = 0.0056X1 + 0.042X2 + 0.0079 (R2 = 0.9109).
Theaflavins
-
Theaflavins not only contribute to the color of tea infusion but also enhance its freshness when combined with caffeine. Figure 2k and l illustrate the dissolution patterns of theaflavins in Se-BF and Se-YL, which align with the general dissolution behavior of solutes. Notably, the dissolution of theaflavins in Se-YL was more temperature-sensitive than in Se-BF. Specifically, the theaflavins content in Se-BF tea infusion was 0.22 mg/100 mL at 100 °C and remained at 0.21 mg/100 mL after 40 min.
Supplementary Table S16 shows the dissolution rates of theaflavins in Se-BF and Se-YL. The dissolution rates of theaflavins in Se-BF at 65, 75, and 85 °C for 40 min were 68.18%, 81.82%, and 90.91%, respectively, while the dissolution rates of theaflavins in Se-YL were 66.67%, 80.95%, and 95.24%, respectively. Comparably, the dissolution rate of theaflavins in Se-YL was faster than Se-BF. Furthermore, Supplementary Table S17 shows the t0.5 of Se-BF at 65, 75, 85, and 100 °C were 17.43, 11.42, 6.78, and 4.53 min, respectively, while the t0.5 of Se-YL were 16.84, 10.90, 6.27, and 4.23 min, respectively, which verified that the dissolution rate of theaflavins in Se-YL was faster than in Se-BF. A stepwise quadratic polynomial regression analysis was conducted on the brewing temperature (X1) and duration (X2) with the concentration of theaflavins (Y) in Se-BF tea infusion yields the regression equation Y = 0.0321X1 + 0.0121X2 − 0.0012 (R2 = 0.9131). The regression equation for the brewing temperature (X1) and duration (X2) with the concentration of theaflavins (Y) extracted from Se-YL tea infusion was Y = 0.0156X1 + 0.0192X2 − 0.0079 (R2 = 0.9239).
Thearubigins
-
Figure 2m and n illustrate the dissolution patterns of thearubigins in Se-BF and Se-YL teas under various brewing conditions, adhering to the general extraction principles of solutes. The dissolution effect of Se-YL was relatively close at 85 and 100 °C, while this phenomenon was not observed in Se-BF. Overall, the dissolution of thearubigins in teas was influenced by both brewing duration and temperature, with the dissolution rate of thearubigins decreasing gradually after the brewing duration exceeded 5 min. Specifically, the dissolution rate of thearubigins was rapid within the first 7 min and then gradually decreased over time. Under the brewing condition of 100 °C for 40 min, the thearubigins content in Se-BF was 4.86 mg/100 mL, while that of Se-YL was 4.12 mg/100 mL.
As shown in Supplementary Table S18, the dissolution rates of thearubigins in Se-BF and Se-YL at 65, 75, and 85 °C for 40 min were 72.39%, 81.28%, 87.01%, and 71.23%, 78.44%, 92.77%, respectively. The dissolution rates of thearubigins were increased by 29.42%, 34.56%, 40.19%, 48.18%, and 27.46%, 35.94%, 42.59%, 45.23% within 0−7 min, and by 42.97%, 46.72%, 46.82%, 51.82%, and 44.77%, 42.50%, 50.18%, 54.77% within 7−40 min ranging from 65 to 100 °C. This phenomenon indicated that the dissolution rate of thearubigins in the Se-YL tea was greater than that in the Se-BF tea during the brewing process. The t0.5 of Se-BF at 65, 75, 85, and 100 °C are 18.38, 12.45, 9.28, and 6.61 min, respectively, while the t0.5 of Se-YL are 16.82, 11.44, 7.79, and 6.40 min, respectively (Supplementary Table S19). This phenomenon indicated that the thearubigins in Se-YL were easier to extract than those in Se-BF. A stepwise regression analysis of the quadratic polynomial was conducted on the brewing temperature (X1), duration (X2), and the concentration of thearubigins (Y) in Se-BF, resulting in the regression equation Y = 0.0074X1 + 0.0025X2 + 0.0022 (R2 = 0.9021). Similarly, for Se-YL, the regression equation was Y = 0.0096X1 + 0.0083X2 + 0.0023 (R2 = 0.9369).
Theabrownins
-
Figure 2o and p illustrate the dissolution pattern of theabrownins in Se-BF and Se-YL, respectively, revealing significant differences in the dissolution pattern of theabrownins at various brewing temperatures over 1 min, which distinctly contrasts with the dissolution behavior of tea polyphenols, soluble total sugar, flavonoids, and thearubigins. Under brewing conditions of 100 °C for 40 min, the contents of theabrownins in Se-BF and Se-YL were respectively 8.20 and 8.01 mg/100 mL.
As shown in Supplementary Table S20, the dissolution rates of theabrownins for Se-BF and Se-YL at 65, 75, and 85 °C for 40 min were 68.59%, 78.66%, 86.16%, and 63.92%, 80.55%, 90.72% respectively. Across the temperature range of 65 to 100 °C, the dissolution rates of theabrownins increased by 39.55%, 56.44%, 70.37%, 81.36% for Se-BF and 28.14%, 44.06%, 58.33%, 70.82% for Se-YL within the first 7 min. Subsequently, from 7 to 40 min, the dissolution rates increased by 29.04%, 22.22%, 15.79%, 18.64% for Se-BF and 35.78%, 36.49%, 32.39%, 29.18% for Se-YL. The t0.5 of Se-BF at 65, 75, 85, and 100 °C were 14.27, 7.10, 3.66, and 1.98 min, respectively, while the t0.5 of Se-YL were 17.88, 9.13, 5.35, and 2.84 min, respectively (Supplementary Table S21). The theabrownins in Se-BF were more easily extracted than those in Se-YL. A quadratic polynomial stepwise regression analysis was performed on the brewing temperature (X1), duration (X2), and the concentration of theabrownins (Y) in Se-BF, resulting in the regression equation Y = 0.003X1 + 0.0025X2 + 0.0039 (R2 = 0.9131). Similarly, for Se-YL, the regression equation was Y = 0.001X1 + 0.0061X2 + 0.0023 (R2 = 0.9245).
Sensory evaluation of tea infusion under different brewing conditions
-
Different brewing conditions affected the dissolution rate of various chemical components in teas, thereby influencing the sensory characteristics of the tea infusion. As depicted in Fig. 3, the sensory evaluation of tea infusions of Se-BF and Se-YL, conducted under varying brewing temperatures and durations, revealed that extended brewing durations intensified the bitterness of the tea infusions. Consequently, the evaluation focused on a brewing duration range of 0 to 15 min. Within the same brewing temperature, the overall sensory evaluation score of the tea infusions increased with the prolongation of brewing duration of up to 5 min, which could be attributed to the harmonious balance between tea polyphenols, free amino acids, soluble sugars, caffeine, and other components. While tea polyphenols and caffeine contribute to the bitter taste of the tea infusion, soluble sugars, and free amino acids enhance its sweetness and umami[8,20]. Nevertheless, beyond the 5-min brewing mark, the continuous dissolution of tea polyphenols surpassed that of soluble sugars, leading to an escalation in the bitter taste of the tea infusions. Meanwhile, the bitter and astringent taste of the tea infusion intensifies, while the fresh and sweet taste diminishes with longer brewing duration, which was consistent with previous studies[20,34,35]. Although raising the brewing temperature might elevate the leaching rate of tea's active ingredients, complex chemical alterations could simultaneously occur at high temperatures. Additionally, the release of tea pigments, notably theabrownins, facilitated a gradual darkening of the tea infusion's appearance and caused the aroma to volatilize and dissipate, ultimately resulting in a decrease in the overall score over time. Comprehensively, the highest overall score achieved was 93.88 for Se-YL, compared to 89.55 for Se-BF, indicating a superior comprehensive quality of Se-YL.
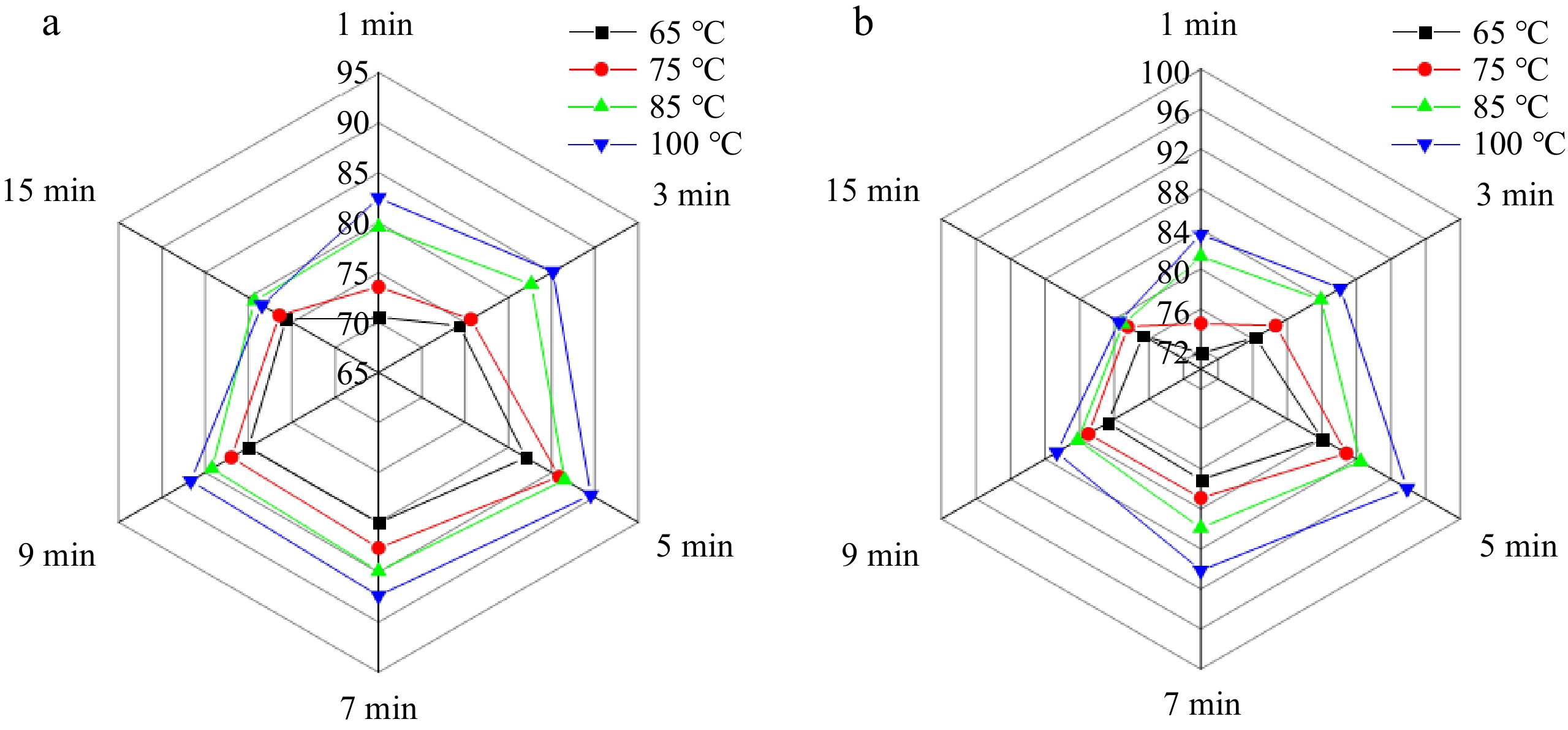
Figure 3.
Radar map of sensory evaluation scores of (a) Se-BF, and (b) Se-YL at different brewing temperatures and duration.
Influence of different brewing times on dissolution of main components
-
As depicted in Fig. 4, an investigation was conducted to assess the impact of brewing times (with the initial three brewings lasting 5 min each, followed by a fourth brewing lasting 1 h) on the dissolution of polyphenols, flavonoids, free amino acids, soluble sugars, and caffeine in roasted green tea (Se-BF) and steamed green tea (Se-YL) at a brewing temperature of 100 °C. The results revealed that the dissolution amounts of all chemical components decreased in both Se-BF and Se-YL as the brewing times increased. The dissolution rates for the first to fourth infusions were separately calculated by the dissolved amounts of the four primary components (Table 1). Notably, the dissolution rates of tea polyphenols, soluble sugars, and caffeine were higher in the first infusion of Se-BF compared to Se-YL. Conversely, the dissolution rates of flavonoids and free amino acids were greater in the first infusion of Se-YL than in Se-BF. Traditionally, the optimal brewing times were varied by different tea varieties. When brewing less than twice, the tea infusion tended to be flavorful and bitter, but the flavor of the tea infusion became milder after the second brewing[36]. For both Se-YL and Se-BF, the third infusion exhibited the lowest dissolution rates for the main components, suggesting that for optimal taste and nutritional qualities, the first and second infusions are preferable when brewing tea.
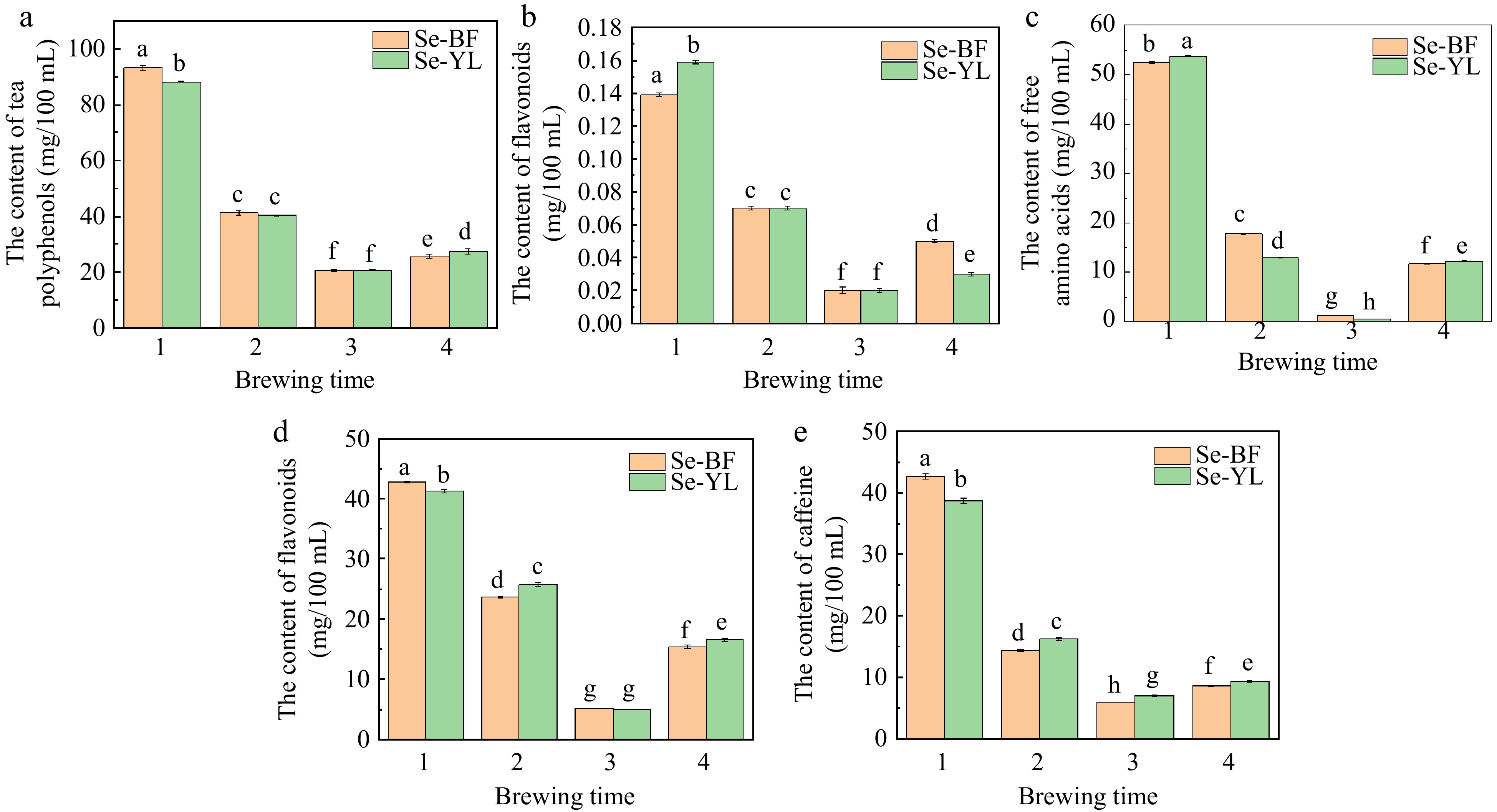
Figure 4.
Influence of different brewing times on the dissolved amount of (a) tea polyphenols, (b) flavonoids, (c) free amino acids, (d) soluble sugars, and (e) caffeine.
Table 1. Influence of brewing time on dissolution rates (%) of main components in Se-BF and Se-YL.
Sample Tea active ingredients Brewing times First infusion Second infusion Third infusion Fourth infusion Se-BF Polyphenols 51.60 22.82 11.38 14.20 Flavonoids 49.82 25.09 7.17 17.92 Free amino acids 63.01 21.36 1.48 14.15 Soluble sugar 49.16 27.20 6.01 17.63 Caffeine 59.61 20.06 8.34 11.99 Se-YL Polyphenols 49.99 22.80 11.66 15.56 Flavonoids 56.99 25.09 7.17 10.75 Free amino acids 67.60 16.33 0.70 15.37 Soluble sugar 46.58 29.08 5.65 18.68 Caffeine 54.27 22.76 9.84 13.13 Effect of different brewing times on in vitro antioxidant activity
-
Figure 5a and b illustrate the antioxidant activities of Se-BF and Se-YL across different brewing times. Notably, the antioxidant activity of the tea infusions peaked during the first brewing and gradually decreased as the brewing time increased. Specifically, the DPPH radical scavenging rate of Se-BF in the first brewing reached 88.68%, which was 1.17, 2.49, and 1.77 times higher than that of the second, third, and fourth brewing, respectively (Fig. 5a). Similarly, the DPPH radical scavenging rate of Se-YL in the first brewing reached 77.69%, surpassing that of the second, third, and fourth brewing by 1.15, 2.44, and 1.83 times, respectively. In terms of the ABTS radical scavenging rate (Fig. 5b), Se-BF in the first brewing achieved 66.59%, which was 1.23, 2.39, and 1.89 times higher than that of the subsequent brewing. Comparable results were observed for Se-YL, with the ABTS radical scavenging rate in the first brewing reaching 67.07%, exceeding that of the second, third, and fourth brewing by 1.27, 2.9, and 1.82 times, respectively. It was noteworthy that the antioxidant capacity of the fourth brewing was higher than the third brewing due to its extended brewing duration of 1 h. Overall, the antioxidant abilities of both tea infusions were enhanced with the increase of dissolved tea active ingredients, particularly tea polyphenols, and the antioxidant capacity of Se-BF tea infusion was consistently higher than that of Se-YL.
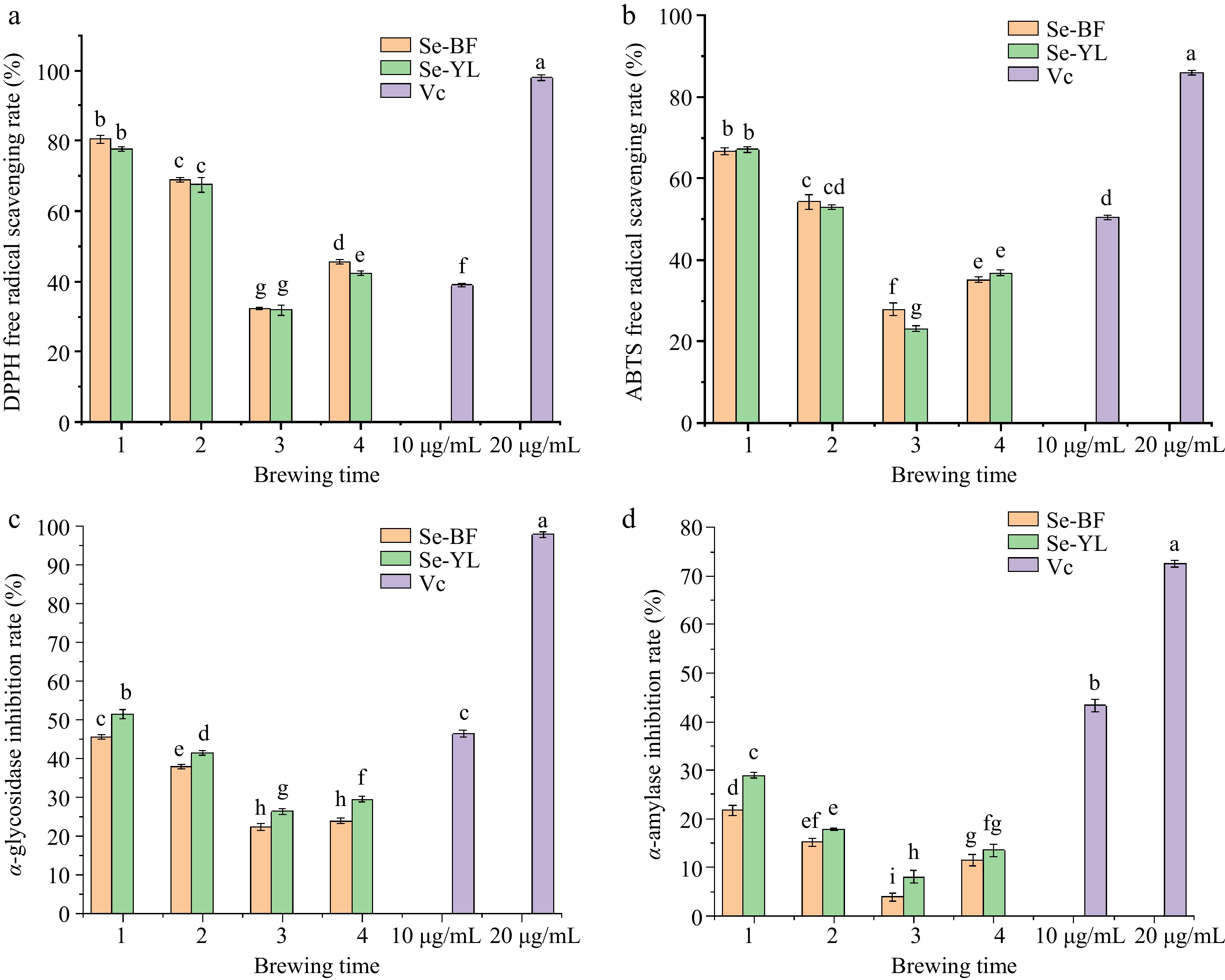
Figure 5.
Effects of different brewing times on (a) DPPH free radical scavenging rate, (b) ABTS free radical scavenging rate, (c) α-glycosidase inhibition rate, and (d) α-amylase inhibition rate of Se-BF and Se-YL.
Effect of different brewing times on in vitro hypoglycemic activity
-
The impact of varying brewing times on the in vitro hypoglycemic activity of Se-BF and Se-YL tea infusions was investigated. Similar to the antioxidant activity, it was evident that the hypoglycemic activity of the tea infusions gradually decreased as the brewing time increased. As illustrated in Fig. 5c, the α-glucosidase inhibition rates of Se-BF and Se-YL in the first brewing were 45.71% and 52.1%, respectively, surpassing the inhibition rates of the subsequent brewing by 1.2 to 2.0 times. Furthermore, the α-amylase inhibition rates of Se-BF and Se-YL in the first brewing were 34.7% and 38.89%, respectively, exceeding those of the subsequent brewing by 1.43 to 5.53 times (Fig. 5d). Although the two types of tea infusions did not exhibit high in vitro hypoglycemic activity, which could be attributed to the low concentration of the tea infusion, it was generally observed that the in vitro hypoglycemic activity of Se-YL tea infusion was superior to that of Se-BF.
-
This study systematically investigated the chemical profiles, dissolution patterns of biochemical components, and in vitro activities of tea infusions with four types of green tea: Selenium-enriched Xiazhou Bifeng (Se-BF), normal Xiazhou Bifeng (BF), selenium-enriched Enshi Yulu (Se-YL), and normal Enshi Yulu (YL). The findings revealed that green tea processing techniques and the application of selenium fertilizer showed significant impacts on the quality components of green teas. By analyzing the dissolution patterns of selenium-enriched green teas under varying brewing temperatures and durations, it was observed that the quantities of dissolved components increased with both the duration and temperature of brewing. Notably, the majority of components in Se-YL were more readily extracted compared to those in Se-BF. Additionally, the present study investigated the impact of brewing frequency on the dissolution of tea components based on a typical daily tea consumption model. The results revealed that the first tea infusion exhibited the highest dissolution amount, accompanied by optimal antioxidant and hypoglycemic activities. Based on sensory evaluations of the tea infusions, the optimal brewing conditions were determined to be 100 °C for 5 min. However, as the brewing time increased, the content of dissolved components in the tea infusions decreased, resulting in a corresponding decline in antioxidant and hypoglycemic activities. Overall, Se-YL demonstrated superior sensory and nutritional qualities compared to Se-BF. Future research endeavors can delve deeper into the comparison between non-enriched and selenium-enriched teas, and the effects of water quality on the dissolution of key tea active components (including volatile components). Furthermore, the molecular mechanisms underlying alterations in tea infusion flavor and in vivo physiological activities should be elucidated in more delicate models.
The authors are grateful for financially sponsorship by Shanghai Agricultural Science and Technology Innovation Project (2023-02-08-00-12-F04598), National Key R&D Program of China (2022YFD2101104), Shaanxi Province key core technology project (2024NC-GJHX-15), National Natural Science Foundation of China (32172223), New Young Teachers Program of Shanghai Jiao Tong University (24X010500154) and China Postdoctoral Science Foundation (BX20220201, 2021M702140).
-
The authors confirm contribution to the paper as follows: conceptualization, writing - draft manuscript preparation: Wei Y; investigation and methodology: Zhang D; writing - manuscript revision: Wei Y, Liang Y, Shi J, Wei X, Wang Y; investigation: Wei K, Peng L; data analysis: Gu H; methodology: Ma P; software and validation: Wang Q, Zhu Z; supervision, funding, administration: Wei X, Wang Y. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Supplementary Table S1 Tea specific information.
- Supplementary Table S2 The content of main chemical components in tea (mg/g).
- Supplementary Table S3 Standard for sensory evaluation of tea infusion.
- Supplementary Table S4 The content of catechins in tea (mg/g).
- Supplementary Table S5 The content of tea pigments (mg/g).
- Supplementary Table S6 Effect of different brewing temperature and duration on the dissolution rate (%) of tea polyphenols in Se-BF and Se-YL.
- Supplementary Table S7
- Supplementary Table S7 Effects of different brewing temperatures on the dissolution time of tea polyphenols in Se-BF and Se-YL.
- Supplementary Table S8 Effect of different brewing temperature and duration on dissolution rate (%) of flavonoids in Se-BF and Se-YL.
- Supplementary Table S9 Effects of different brewing temperatures on dissolution duration of flavonoids in Se-BF and Se-YL.
- Supplementary Table S10 Effect of different brewing temperature and duration on dissolution rate (%) of soluble sugar in Se-BF and Se-YL.
- Supplementary Table S11 Effects of different brewing temperatures on dissolution duration of soluble sugars in Se-BF and Se-YL.
- Supplementary Table S12
- Supplementary Table S12 Effect of different brewing temperature and duration on dissolution rate (%) of free amino acids in Se-BF and Se-YL.
- Supplementary Table S13 Effects of different brewing temperatures on dissolution duration of free amino acids in Se-BF and Se-YL.
- Supplementary Table S14 Effect of different brewing temperature and duration on dissolution rate (%) of caffeine in Se-BF and Se-YL.
- Supplementary Table S15 Effects of different brewing temperatures on dissolution duration of caffeine in Se-BF and Se-YL.
- Supplementary Table S16 Effect of different brewing temperature and duration on dissolution rate (%) of theaflavins in Se-BF and Se-YL.
- Supplementary Table S17 Effects of different brewing temperatures on dissolution time of theaflavins in Se-BF and Se-YL.
- Supplementary Table S18 Effect of different brewing temperature and duration on dissolution rate (%) of thearubigins in Se-BF and Se-YL.
- Supplementary Table S19 Effects of different brewing temperatures on dissolution duration of thearubigins in Se-BF and Se-YL.
- Supplementary Table S20 Effect of different brewing temperature and duration on dissolution rate (%) of theabrownins in Se-BF and Se-YL.
- Supplementary Table S21 Effects of different brewing temperatures on dissolution duration of theabrownins in Se-BF and Se-YL.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wei Y, Zhang D, Liang Y, Shi J, Wei K, et al. 2024. Chemical profiles, dissolution patterns, and in vitro bioactivities of selenium-enriched green teas: impact of brewing conditions. Food Innovation and Advances 3(4): 372−384 doi: 10.48130/fia-0024-0035
Chemical profiles, dissolution patterns, and in vitro bioactivities of selenium-enriched green teas: impact of brewing conditions
- Received: 27 August 2024
- Revised: 11 November 2024
- Accepted: 11 November 2024
- Published online: 27 November 2024
Abstract: The dissolution patterns of different teas determine the sensory quality and health attributes of the tea infusion. In this study, the chemical profiles of two typical selenium-enriched green teas, Xiazhou Bifeng (Se-BF), Enshi Yulu (Se-YL), and their corresponding regular green teas (BF and YL) were determined. Under the application of selenium fertilizer, the contents of caffeine, polyphenols, and gallic acid decreased, while the contents of theaflavins, theabrownins, and chlorophylls increased. The selenium content in BF and YL is 0.05−0.16 mg/kg and 0.33−0.43 mg/kg respectively, while after the application of exogenous selenium, the selenium content in Se-BF and Se-YL reached 1.28 to 2.17 mg/kg and 0.37−2.23 mg/kg respectively. The dissolution patterns of Se-BF and Se-YL were investigated under different brewing conditions (temperature and duration), and the main components of Se-YL were more easily dissolved out than Se-BF, which might be attributed to the steaming process of Se-YL. Based on the sensory evaluation of tea infusion, 100 °C and 5 min were the optimal brewing conditions. Based on a daily tea consumption model, the increased brewing time reduced the content of dissolved components in tea infusions, along with the decreased in vitro antioxidant and hypoglycemic activities. Collectively, Se-YL demonstrated superior sensory and nutritional attributes compared to Se-BF. This study explored the influence of brewing conditions on the dissolution patterns and in vitro bioactivities of selenium-enriched green teas, providing guidance for scientific tea brewing and consumption.













