-
Auricularia auricula is one of the four major cultivated edible fungi globally and ranks second in China in terms of production. It is rich in functional components such as carbohydrates, proteins, polysaccharides, and polyphenols, making it a medicinal and edible fungus[1,2]. Auricularia auricula polysaccharides (AAP), as the main active components in Auricularia auricula, have biological effects such as antioxidant[3], antitumor[4], hypolipidemic[5], anti-inflammatory[6], and immunomodulatory[7]. Due to its high biocompatibility, biodegradability, safety, and low cost, AAP has been thoroughly investigated as a carrier for drug delivery. The high water-holding capacity and the capability to create hydrophilic colloidal films on oil droplet surfaces via van der Waals forces, attributed to the polyhydroxy structure of AAP, preventing droplet aggregation and enhancing emulsification capacity and stability[8]. Additionally, the active aggregation of oil droplets, which is induced by Brownian motion, can be mitigated by the high viscosity of an aqueous AAP solution[9], making it a promising natural food emulsifier.
The extraction method significantly influences the structure of AAP, which in turn affects its emulsifying properties. For instance, water extraction causes minimal structural changes to the polysaccharides, whereas acid extraction results in degradation and the release of more acidic components[10]. Ultrasound can break down the side chains of polysaccharides, thereby enhancing the proportion of galactoglucans to glucose[11]. Meanwhile, microwave-assisted extraction can decrease both the molecular weight and the degree of methyl esterification
(DM)[12]. Polysaccharides are extracted in different ways depending on their sources. Extraction methods commonly employed encompass hot water extraction, acid-base extraction, ultrasound-assisted extraction, and enzymatic extraction, etc.Emulsions are an effective system for delivering bioactive components, protecting the delivery of nutrients and improving the absorption efficiency of lipid-soluble substances, but their instability limits the application of emulsions[13]. Ensuring the steady state of emulsions is crucial for the effective delivery of active ingredients[14], and emulsifiers are a key factor in maintaining emulsion stability. The presence of hydrophobic groups, including methoxy and acetyl groups, enables polysaccharides to function as efficient emulsifiers in water-in-oil systems, promoting swift adsorption onto the surface of oil droplets[15]. The hydrophilic regions, along with the side chains and main chains of the polysaccharide, protrude into the continuous phase, thereby creating spatial resistance and electrostatic repulsion that hinder or decelerate the aggregation of oil droplets[16]. Consequently, this mechanism maintains the stability of the emulsion[17]. Compared to other polysaccharides, AAP has a higher molecular weight, which helps increase the viscosity, inhibiting the motion and clustering of droplets within the emulsion system, thus further enhancing its stability[18]. Additionally, the antioxidant and antibacterial properties of the polysaccharide contribute to the long-term storage stability of the emulsion. In the production process, both the pH level and the existence of ions influence the behavior of polysaccharide emulsions[13]. Ions can modify the characteristics of polysaccharides, a crucial aspect for understanding the fundamental mechanisms that contribute to emulsion stability[19]. Nonetheless, the influence of ionic strength on the emulsifying capabilities of AAP has not been extensively studied. Consequently, gaining an understanding of the interplay between AAP's physical and chemical attributes, its interfacial behavior, and emulsifying performance could offer valuable perspectives on its potential as a natural emulsifier.
β-carotene serves as a natural colorant and antioxidant in food products, yet its application within the food industry is significantly restricted due to its low solubility in water and chemical instability[20]. It has been found that the bioavailability of carotenoids can be relatively increased when they are consumed together with other lipids[21]. Emulsification could potentially serve as an efficacious approach to improve the bioavailability of β-carotene[22]. Hence, the development of AAP-W emulsion systems containing β-carotene can enhance the chemical stability of the functional component, shield the active substance from degradation, and additionally boost its bioavailability. In this study, three kinds of Auricularia auricula polysaccharides were obtained by hot water extraction (AAP-W), hot acid extraction (AAP-A), and hot alkali extraction (AAP-AL), respectively. The AAP-W, which exhibited the highest emulsification stability, was chosen to formulate oil-in-water emulsions, and subsequently, an AAP-W emulsion system incorporating β-carotene was developed. This system improved the retention, bioavailability, and digestive stability of β-carotene. It also broadened the application prospects of Auricularia auricula polysaccharides and offered a novel strategy for the creation of innovative functional foods.
-
Auricularia auricula was purchased from Dongning Linxiang Shanzhen Products Processing Co., Ltd. (Heilongjiang, China). Corn germ oil was purchased from Shandong Samsung Corn Industry Technology Co., Ltd. (Shandong, China). β-carotene (purity ≥ 95%) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Enzymes and coenzymes were purchased from Shanghai Solaibao Biotechnology Co., Ltd., and other chemical reagents were obtained from Tianjin Kaitong Chemical Reagent Co., Ltd. All experimental reagents were of analytical grade.
Extraction of AAP
-
AAP are extracted by three methods: hot water extraction, acid extraction, or alkali extraction[23]. The comprehensive procedures are outlined in the Supplementary File 1. The yields of AAP were calculated as follows:
$\begin{array}{l} Auricularia\;auricula\;polysaccharide\;({\text{%}}) = \\\dfrac{{Auricularia\;auricula\;polysaccharide\;dry\;weight\;(g)}}{{Auricularia\;auricula\;dry\;weight\;(g)}}\end{array} $ Physicochemical properties of AAP
Total sugar content of AAP
-
The phenol-sulfuric acid method, slightly modified, was utilized to determine the overall sugar content in AAP according to the standard NY/T 1676-2008[8]. The comprehensive procedures are outlined in the Supplementary File 1.
Protein content of AAP
-
The protein content in AAP was assessed utilizing the Coomassie brilliant blue methods[24]. The comprehensive procedures are outlined in the Supplementary File 1.
Alduronic acid content
-
With minor adjustments, the content of alduronic acid in AAP was determined by referencing the method outlined by Taylor & Buchanan-Smith[25]. The comprehensive procedures are outlined in the Supplementary File 1.
Molecular weight distribution of AAP
-
The determination of AAP's molecular weight was carried out through gel permeation chromatography (GPC). The detection process involved the use of a TSKGEL GMPWXL aqueous gel chromatography column (dimensions: 7.8 mm × 300 mm; TSK-gel PWXL G4000, TOSOH (TSK), Japan) along with a RID-20 differential refractive index detector. For the calibration of the GPC system, polyethylene oxide (PEO) was utilized.
Monosaccharide composition of AAP
-
The monosaccharide composition of AAP was ascertained through high-performance liquid chromatography (HPLC)[26]. The detection was executed using an HPLC system (model U300; Thermo Fisher Scientific, MA, USA) equipped with an Xtimate C18 chromatographic column. To compute the molar mass of each monosaccharide, the peak area ratios were determined using the area normalization method. Comprehensive procedures can be found in the Supplementary File 1.
Scanning electron microscopy (SEM)
-
The microstructure of AAP was examined using a field emission scanning electron microscope (model JSM-6480A, manufactured by JEOL Ltd., Tokyo, Japan)[27].
Fourier transform infrared (FT-IR) spectroscopic analyses
-
The structure of AAP was analyzed and identified by FT-IR according to Wu et al.[28]. Comprehensive procedures can be found in the Supplementary File 1.
Thermogravimetric (TG) analysis
-
The TG analysis of AAP was measured using the method of Kazemi et al.[29] with slight modifications. Comprehensive procedures can be found in the Supplementary File 1.
X-ray diffraction (XRD)
-
The crystallization properties of AAP were determined by XRD[30,31]. Comprehensive procedures can be found in the Supplementary File 1.
Three-phase contact angle
-
The three-phase contact angle of AAP was measured using an optical contact angle meter manufactured by Powereach Instruments Ltd. (Shanghai, China). Comprehensive procedures can be found in the Supplementary File 1.
Emulsifying properties of AAP
-
After preparing the emulsion, the absorbance was measured at 500 nm with a spectrophotometer and the EAI and ESI of the emulsion were calculated according to the following equations:
$ EAI({m^2}{g^{ - 1}}) = \dfrac{{2 \times 2.303 \times {A_0} \times DF}}{{C \times \phi \times \theta \times 1000}}EAI({m^2}g) $ $ ESI(\min ) = \dfrac{{{A_0} \times 10}}{{\Delta A}} $ where, DF: dilution times, C: polysaccharide concentration (g/mL), φ: oil phase volume fraction, θ: light range of cuvette (cm), ΔA: |A0−A10|, A0: the absorbance measured by the above method at 0 min of emulsion preparation, A10: the absorbance measured by the above method at 10 min of emulsion preparation.
Effect of different conditions on the stability of AAP-W stabilized emulsions
-
The effects of polysaccharide concentration, oil-water ratio, pH value and salt ion concentration on emulsion stability were investigated. Comprehensive procedures can be found in the Supplementary File 1.
Characterization of AAP emulsion
Resting stability
-
A 10-ml transparent glass bottle with a lid was used to store the prepared emulsion. Observations were made at room temperature to note any appearance changes in the emulsion, and photographs were taken for record-keeping purposes.
Grain size and zeta potential
-
The particle size of the emulsion was measured utilizing a laser particle size analyzer (model LS13 320, manufactured by Beckman Coulter, USA). Additionally, the zeta potential of the emulsion was assessed using a Zetasizer-Nano-ZS laser particle size analyzer.
Rheological properties
-
A modular intelligent rheometer (AR2000ex, TA Instruments, USA) was used to measure the rheological properties of the emulsion. The stress was set at 1 Pa and the shear rate ranged from 0.1 to 100 s−1. The frequency scanning range was 0.1−100 rad·s−1.
AAP emulsion loaded with β-carotene
Preparation of β-carotene AAP emulsion
-
A certain amount of β-carotene was weighed and dissolved in 10 ml of corn oil, stirring evenly until it is completely dissolved, ensure that the final concentration of β-carotene is 0.1% (w/v) and exposure to light was avoided during the entire process. Next, an amount of AAP-W was measured and dissolved it in 10 ml of deionized water containing 0.02% sodium azide, resulting in a polysaccharide solution with a final concentration of 1%. Then, the oil phase (containing β-carotene) was mixed with the aqueous phase (containing AAP-W polysaccharide solution) and the mixture dispersed at 14,000 rpm using a high-speed disperser for 3 min. As a result, an oil-in-water emulsion loaded with β-carotene was obtained.
Characterization of β-carotene loaded AAP emulsions
-
Characterization of β-carotene loaded AAP emulsions Resting stability A 10-ml transparent glass bottle with a lid was used to store the prepared emulsion. Observations were made at room temperature to note any appearance changes in the emulsion, and photographs were taken for record-keeping purposes. Grain size and Zeta potential The particle size of the emulsion was measured utilizing a laser particle size analyzer (model LS13 320, manufactured by Beckman Coulter, USA). Additionally, the zeta potential of the emulsion was assessed using a Zetasizer-Nano-ZS laser particle size analyzer. Rheological properties A modular intelligent rheometer (AR2000ex, TA Instruments, USA) was used to measure the rheological properties of the emulsion. The stress was set at 1 Pa and the shear rate ranged from 0.1 to 100 s−1. The frequency scanning range was 0.1−100 rad·s−1.
Retention of β-carotene
-
β-carotene was dissolved in chloroform and prepared into solutions with concentrations of 0.5, 1, 2, 3, 4, and 5 μg·mL−1. The absorbance was measured at a wavelength of 450 nm, and based on these measurements, the standard curve for β-carotene was drawn. The retention rate of β-carotene was determined using the accelerated oxidation method[32]. Comprehensive procedures can be found in the Supplementary File 1.
In vitro digestion of beta-carotene-loaded emulsion
-
In vitro simulated digestion experiments were performed in accordance with the methods of Zhang et al.[33], with slight modifications. Comprehensive procedures for these experiments can be found in the Supplementary Materials. Bioaccessibility (B*) and Digestive Stability (S*) of β-carotene were calculated using the following formula:
$ {B^ * } = \dfrac{{{C_{Micelle}}}}{{{C_{Digesta}}}} \times 100 $ $ {S^ * } = \dfrac{{{C_{Digesta}}}}{{{C_{Inital}}}} \times 100 $ where, CMicelle: concentration of β-carotene in micelles. CDigesta: concentration of β-carotene in the mixture after digestion in the small intestine stage. CInitial: starting concentration of β-carotene in emulsions.
Statistical analysis
-
SPSS software was used to conduct a statistical analysis of all the data. The results presented are the average of at least three trials, expressed as 'mean ± SD'. A one-way ANOVA, coupled with Duncan's test, was employed to identify significant differences. Throughout the study, the significant level was set as p < 0.05.
-
As shown in Table 1, AAP-AL had the highest yield, while AAP-W had the lowest yield. Alkaline extraction yielded a higher polysaccharide content compared to water extraction, as it can break down the cell walls and release bound polysaccharides, while water extraction can only dissolve water-soluble polysaccharides[23]. Similarly, the protein content of AAP-W was notably greater than that of AAP-A and AAP-AL, suggesting that acid and alkaline can break down some proteins in Auricularia auricula, resulting in a lower content of extracted proteins[23]. The alduronic acid content of AAP-A was significantly greater than that of AAP-W and AAP-AL, potentially due to the acid hydrolysis of some glycosidic bonds connecting alduronic acids, or the removal of proteins or metal salt ions that may be bound to alduronic acids and reduce their solubility[34].
Table 1. Yield, total sugar, protein, and alduronic acid content of AAP extracted by different methods.
Yield (%) Total sugar (%) Protein (%) Alduronic acid (%) AAP-W 3.04 ± 0.90a 66.22 ± 0.15a 8.98 ± 1.26a 12.62 ± 0.17b AAP-A 1.31 ± 0.72b 59.69 ± 0.22c 5.04 ± 0.60b 13.23 ± 0.24a AAP-AL 3.78 ± 0.53a 61.48 ± 0.28b 5.48 ± 0.20b 10.13 ± 0.17c Identical letters denote no significant differences, whereas differing letters signify significant differences at p < 0.05. Molecular weight analysis
-
As shown in Table 2, the number-average molecular weights (Mn) value of AAP-W exceeded the values of both AAP-A and AAP-AL. The potentially lower molecular weights of AAP-A and AAP-AL could be attributed to the degradation of the polysaccharide, which occurs as a result of acid-base cleavage of their glycosidic bonds[35]. In addition, the polydispersity coefficient of AAP-W was closest to 1, indicating a good degree of homogeneity.
Table 2. Molecular weight and polydispersity coefficient of AAP under different extraction methods.
Mn (Da) Mw (Da) Mw/Mn AAP-W 3.53 × 105 ± 510a 6.72 × 105 ± 360b 1.90 ± 0.4b AAP-A 1.72 × 105 ± 380c 1.01 × 106 ± 310a 5.89 ± 0.9a AAP-AL 3.16 × 105 ± 490b 6.56 × 105 ± 450c 2.07 ± 0.5b Identical letters signify no significant differences, while differing letters indicate significant differences at a significance level of p < 0.05. Monosaccharide composition analysis
-
Table 3 illustrates that the method of extraction does not alter the types of monosaccharide components within AAP; all samples exhibit a consistent monosaccharide profile, predominantly featuring mannose and glucose. However, the molar ratios of these constituent sugars vary significantly among different extraction techniques. This observation reaffirms AAP's classification as a heteropolysaccharide. Notably, AAP also contains a minor proportion of arabinose (Ara). Despite its low abundance, this constituent plays a role in the development of neutral sugar side chains, which may have an impact on the emulsifying properties of the polysaccharide[36].
Table 3. Monosaccharide composition of AAP under different extraction methods.
Monosaccharide composition (%) AAP-W AAP-A AAP-AL Man 38.369 35.227 39.243 Rib 2.581 2.022 2.107 Rha 0.217 0.311 0.258 GlcA 4.787 4.45 4.193 GalA 0.133 0.144 0.142 Glc 46.76 49.124 44.479 Gal 2.217 3.481 3.272 Xyl 1.203 1.913 1.957 Ara 0.271 0.167 0.992 Fuc 3.462 3.16 3.356 SEM analysis
-
Figure 1a displays the surface morphology and structural characteristics of AAP. AAP-W exhibited an irregular lamellar structure with large cracks in the center. This may be because water-soluble polysaccharides form large lamellar structures in water and shrink and crack during the drying process[37]. Similar structures have been observed in groundnut root polysaccharides[38]. AAP-A showed a fish scale-like appearance, presumably because acid decreases the molecular weight and viscosity of the polysaccharide, facilitating its precipitation and drying[39]. AAP-AL is filamentous, possibly due to the alkaline solution increasing the charge density and solubility of the polysaccharides, allowing them to stretch and become filamentous[40].
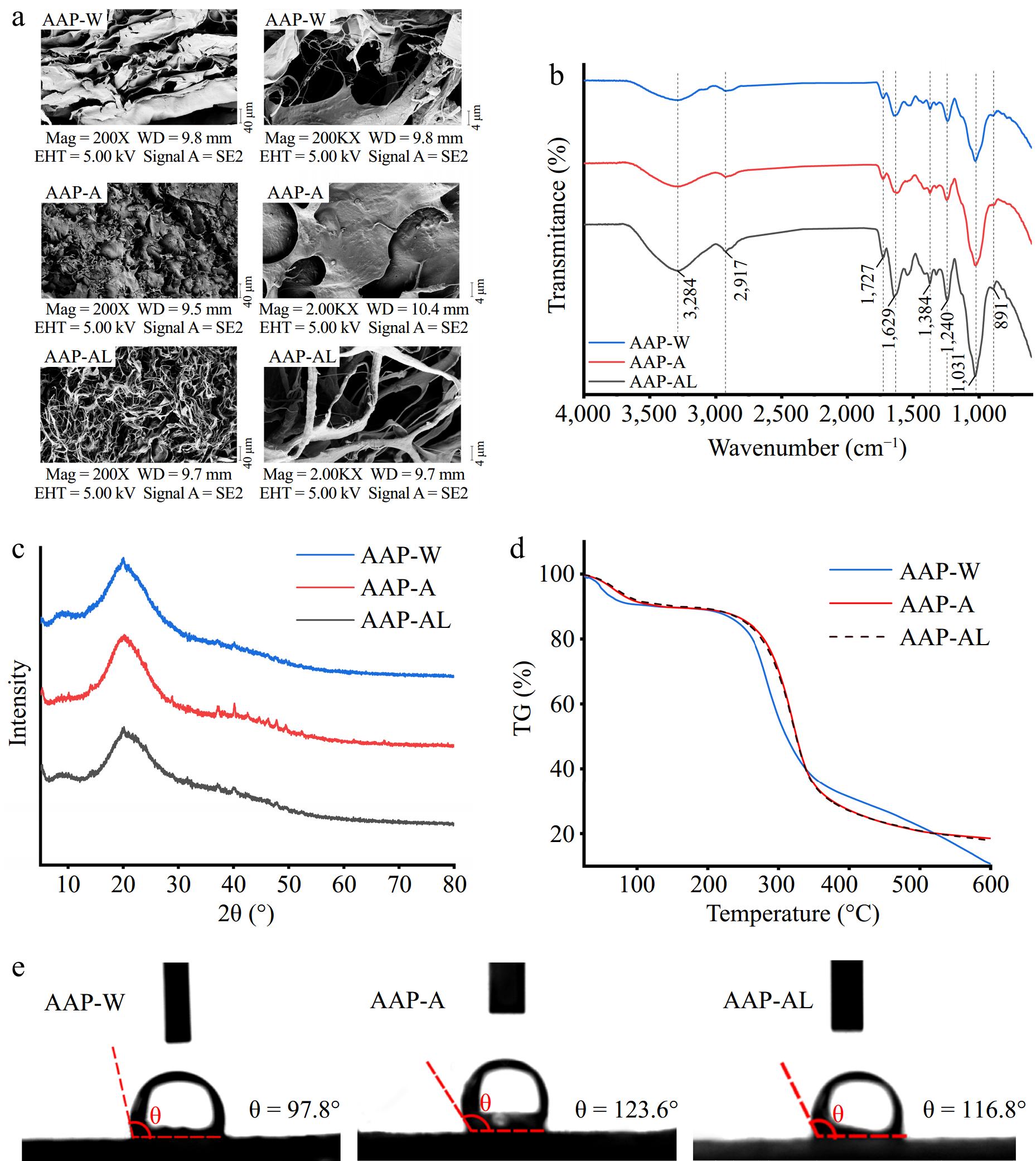
Figure 1.
Physicochemical properties and structural characterization of AAP. (a) SEM. (b) FI-IR. (c) TG. (d) XRD. (e) Three-phase contact angle.
FI-IR analysis
-
Figure 1b revealed characteristic absorption peaks in the functional group region (4,000−1,400 cm−1). At around 3,284 cm−1, there was an absorption peak indicating the O-H stretching vibration, suggesting the presence of typical carbohydrate features in AAP. The absorption peak near 2,917 cm−1 corresponded to C-H stretching vibrations, while the peak around 1,629 cm−1 corresponded to the asymmetric stretching vibration of C=O in the polysaccharide structure. The absorption peak near 1,240 cm−1 may be attributed to the C-O-C vibration, and the peak near 891 cm−1 suggested the presence of β-glycosidic bonds in their molecular structure[41]. All three spectra showed the typical characteristic absorption peaks linked to polysaccharides, indicating that the various extraction techniques did not compromise the fundamental structure of the polysaccharides.
TG analysis
-
The TC curves of the three polysaccharides are depicted in Fig. 1c, and they all exhibit similar patterns. All three polysaccharides undergo an initial weight loss of approximately 10% within the temperature range of 25−150 °C, primarily due to the evaporation of absorbed and bound water within the polymers[42]. A second significant weight loss occurs between 200−400 °C, with a weight loss rate of approximately 60%, which is attributed to the thermal decomposition of the polysaccharides. Generally, polysaccharides remain stable below 200 °C and exhibit thermal stability.
XRD analysis
-
Figure 1d showed that in the measurement range from 5° to 80°, there is only a broad diffraction peak around 20° and no obvious diffraction peak in other detection ranges, indicating that all three were in an amorphous state and non-crystalline structure. The various extraction techniques do not alter the amorphous characteristic of polysaccharides, which arises from the hydrogen bonding interactions among the hydroxyl groups within the polysaccharide chains.
Three-phase contact angle analysis
-
As shown in Fig. 1e, the contact angle of AAP-W is 97.8°, which is closer to 90° in comparison, indicating that the wettability of the AAP-W surface to the liquid is closer to neutral, which is more favorable to regulate the distribution and migration of liquid droplets in the emulsion, thus improving the stability of the emulsion. In general, the smaller the contact angle, the better the surface wettability of the liquid to the solid, and as an emulsifier for preparing emulsions requires stability at the interface between the aqueous and oil phases[43,44].
Analysis of emulsification stability of AAP
-
Figure 2a showed that the emulsions prepared by AAP-W and AAP-AL remained homogeneous without water and oil emission after one month of placement. Figure 2b demonstrated that the emulsification activity index (EAI) and emulsion stability index (ESI) of AAP-W were significantly higher than those of AAP-A and AAP-AL. Prior research indicates that polysaccharides characterized by a lower molecular weight (Mw) tend to display diminished viscosity and swifter migration rates. Consequently, these attributes lead to a more rapid and pronounced decline in interfacial tension[45] indicating that AAP-W exhibited stronger emulsification stability.
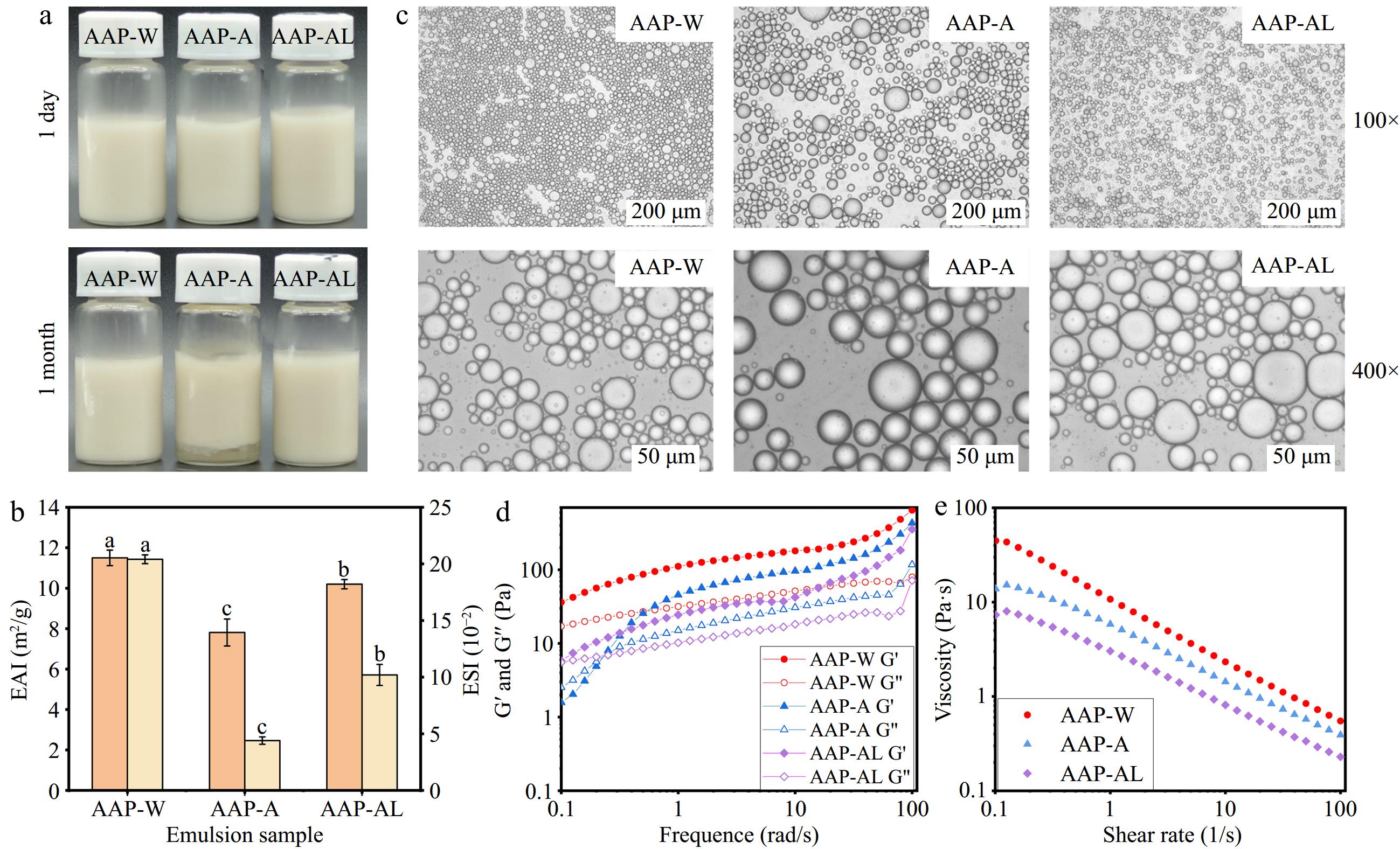
Figure 2.
Emulsification properties of AAP. (a) Emulsion prepared from AAP. (b) EAI and ESI. (c) Micromorphology of AAP emulsion. (d) Rheological dynamic analysis of AAP. (e) Rheological static results of AAP.
Figure 2c shows the microscopic morphology of the AAP emulsions: the emulsions prepared by AAP-A and AAP-AL had larger droplets and were not uniformly dispersed, and the emulsions prepared by AAP-W had the smallest droplets. The capacity of AAP-W to quickly adsorb onto the surface of droplets and promptly reduce interfacial tension enables the formation of smaller droplets with a more uniform size distribution[46]. The results indicated that the emulsions prepared with AAP-W demonstrated the greatest stability.
As depicted in Fig. 2d, the loss modulus (G') of AAP-W-based emulsions was consistently greater than the storage modulus (G") across the entire frequency range, indicating that AAP-W-based emulsions possessed solid-like properties and were more stable. Figure 2e demonstrated that AAP-W-based emulsions had the highest viscosity in the scan range, The observed reduction in the viscosity of AAP-A and AAP-AL can be ascribed to an increase in emulsion droplet size, which leads to a decreased concentration of polysaccharide molecules per unit volume. This lower density weakens the interactions among polysaccharides, thereby reducing overall viscosity[47]. Indeed, elevated viscosities tend to restrict particle motion, confining the mobility of emulsion droplets and impeding their free flow throughout the system. Moreover, high viscosity not only slows down but also inhibits droplet coalescence, playing a crucial role in maintaining the emulsion's structure and long-term stability[48]. In summary, AAP-W exhibited superior emulsifying properties compared to AAP-A and AAP-AL, making it the preferred choice for further research.
Effect of different factors on the stability of AAP-W emulsion
Effect of AAP-W concentration on the stability of emulsions
Effect of AAP-W concentration on the resting stability of emulsions
-
Figure 3a illustrates the emulsions that were formulated using varying concentrations (0.1%−3%, w/v) of AAP-W. After 30 d of standing, the emulsions made with AAP-W concentrations of 0.1%, 0.25%, and 0.5% exhibited varying degrees of phase separation. This phenomenon was mainly attributed to inadequate coverage of the oil-water interface when the polysaccharide concentration was excessively low. However, emulsions prepared with AAP-W concentrations of 1%−3% remained uniformly stable after 30 d. This could be ascribed to the conversion of the emulsion into an emulsion gel after a specific duration of resting, which further augments its stability during prolonged storage. In contrast to emulsions, emulsion gels exhibit semi-solid physical characteristics, offering enhanced protection and immobilization of functional components[49]. The findings revealed that, within a certain range, the higher the concentration of AAP-W, the greater the stability of the emulsions, with a stable state being maintained when the AAP-W concentration attained 1%.
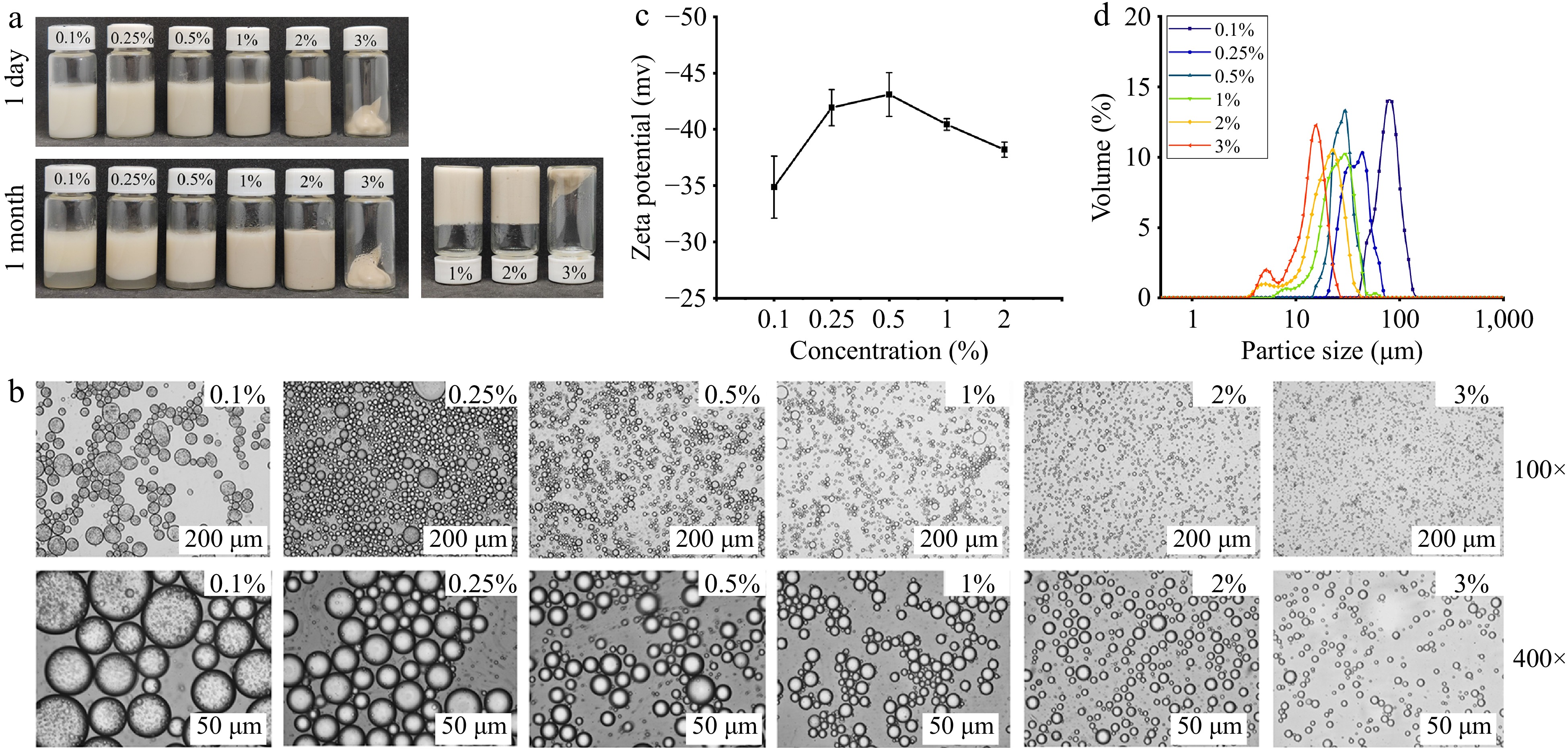
Figure 3.
Effect of AAP-W concentration on the stability of emulsions. (a) Static stability of emulsion. (b) Micromorphology of emulsion. (c) Zeta potential. (d) Particle size distribution of emulsion.
Effect of AAP-W concentration on the zeta potential of emulsions
-
As shown in Fig. 3c, the measured zeta potentials were all negative, which may be due to the polysaccharide itself having a negative charge or dissociating negatively charged groups in water, such as carboxyl groups, sulfate groups, etc.[50]. As the concentration of AAP-W in the emulsion rose, the absolute value of the emulsion's potential initially increased and subsequently declined, but the decrease was significantly smaller than the increase, and the absolute zeta potential value for all emulsions exceeded 30, suggesting that the emulsions exhibited relative stability across the concentration range spanning from 0.1% to 2%. A low zeta potential value predisposes the emulsion to flocculation, whereas a high zeta potential ensures sufficient electrostatic repulsion to counteract inter-droplet interactions[15].
Effect of AAP-W concentration on the microscopic morphology and particle size of emulsions
-
Figure 3b showed the microscopic morphology of the emulsion droplets at varying AAP-W concentrations ranging from 0.1% to 3%. The droplet diameter was largest when the AAP-W concentration was 0.5%, as the polysaccharide concentration increased, the droplet size decreased, reaching its smallest when the concentration was 3%. Higher polysaccharide concentrations can stabilize a larger surface area during preparation and help the emulsifier cover the droplet surface more rapidly, thereby better-preventing droplet aggregation and resulting in smaller emulsion particle sizes[51].
To conduct a more thorough observation of the variations in emulsion droplet size, the emulsion particle size was analyzed (Fig. 3d). The findings indicated that elevating the polysaccharide concentration within the range of 0.1% to 3% led to a decrease in the size of the oil droplets. This could potentially be attributed to the increased adsorption of polysaccharides at the oil-water interface, which facilitates the formation of smaller oil droplets[52], and similar observations were reported by Meng et al.[53].
Effect of oil phase volume fraction on emulsion stability
Effect of oil phase volume fraction on the resting stability of emulsions
-
Figure 4a shows the emulsions prepared under different oil phase volume fractions (φ = 0.2−0.7) with a 1% concentration of AAP-W. It can be observed that the appearance of the emulsions remains largely consistent within the range of φ = 0.2−0.6. At φ = 0.7, a small amount of phase separation and color unevenness can be seen in the emulsion. It is worth noting that as the volume fraction of the emulsified phase (φ) increases, there comes a point where the oil phase is sufficient to destabilize the emulsion, leading to delamination at higher φ values, rather than lower[54,55]. However, this phenomenon did not occur in the stable emulsion prepared with AAP-W, indicating that AAP-W has excellent interfacial adsorption ability. Similar results were found by Xiao et al.[56].
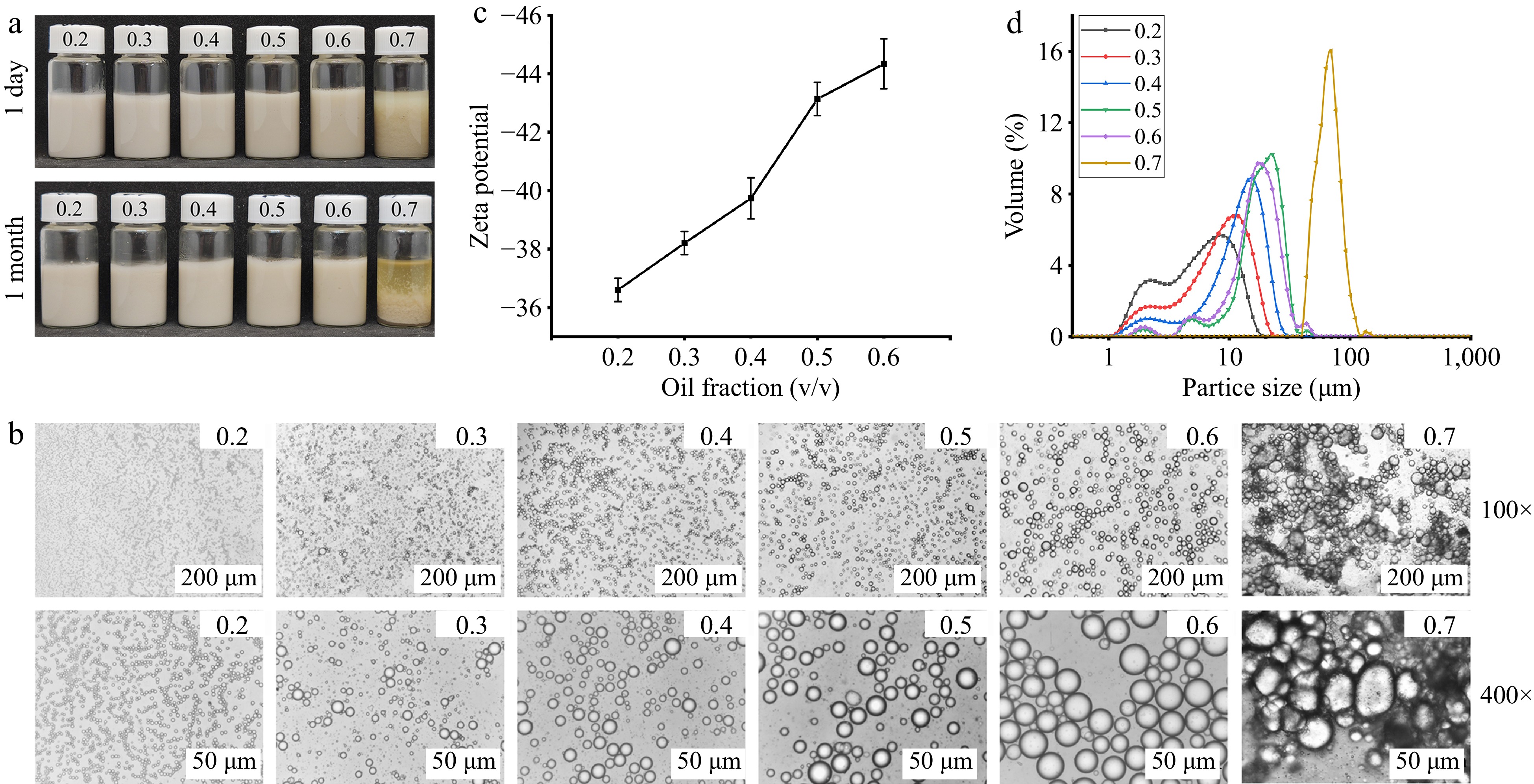
Figure 4.
Effect of oil phase volume fraction on the resting stability of emulsions. (a) Static stability of emulsion. (b) Micromorphology of emulsion. (c) Zeta potential. (d) Particle size distribution of emulsion.
Effect of oil phase volume fraction on the zeta potential of the emulsion
-
Figure 4c showed that the zeta potential is negative in the variation range of φ = 0.2−0.6. The absolute value of the zeta potential rises as the volume of the oil phase increases, and all of them are greater than 30, indicating that the emulsions are all in a stable state[57,58].
Effect of oil phase volume fraction on the microscopic morphology and particle size of the emulsion
-
Figure 4b illustrates that within the range of φ = 0.2−0.6, the distribution of emulsion droplets was fairly uniform, enabling the emulsion to maintain good stability. However, as φ increased, the droplet size increased notably. This phenomenon can be attributed to the insufficiency of AAP-W to fully stabilize the oil phase under high oil phase conditions, causing the emulsion to stabilize as much of the oil phase as possible by increasing the droplet size. Comparable findings were reported by Huang et al.[59].
Figure 4d provided a clearer visual representation, showing that the particle size enlarges with an increase in φ. This outcome can be ascribed to the fact that the overall volume of the emulsion remained constant (20 mL). As the oil phase increased, the volume of the aqueous phase correspondingly decreased, and often, there was not enough AAP-W to adsorb at the oil-water interface, which consequently led to the formation of larger droplets[60].
Effect of pH on the stability of emulsions
-
Figure 5a shows the emulsions prepared at different pH values (pH = 3, 5, 7, 9, 11) for AAP-W concentration of 1% and φ = 0.5, all of which can maintain a stable state. Figure 5b & c demonstrated that the emulsions formulated with AAP-W possessed outstanding pH stability. This could potentially be attributed to the creation of a three-dimensional network of oil droplets, enhancing the emulsions' resistance to changes in pH[57]. However, the particle size of the droplet varies at different pH levels. Under weak acidic conditions, the conformation of the polysaccharide relaxes, resulting in a decreased adsorption rate onto the oil droplets and subsequently larger droplet formation. As the pH approaches the polysaccharide's isoelectric point (pH 3.5), AAP molecules exhibit a tendency to partially aggregate, leading to an irregular particle size distribution and the emergence of complexes of varying sizes[17].
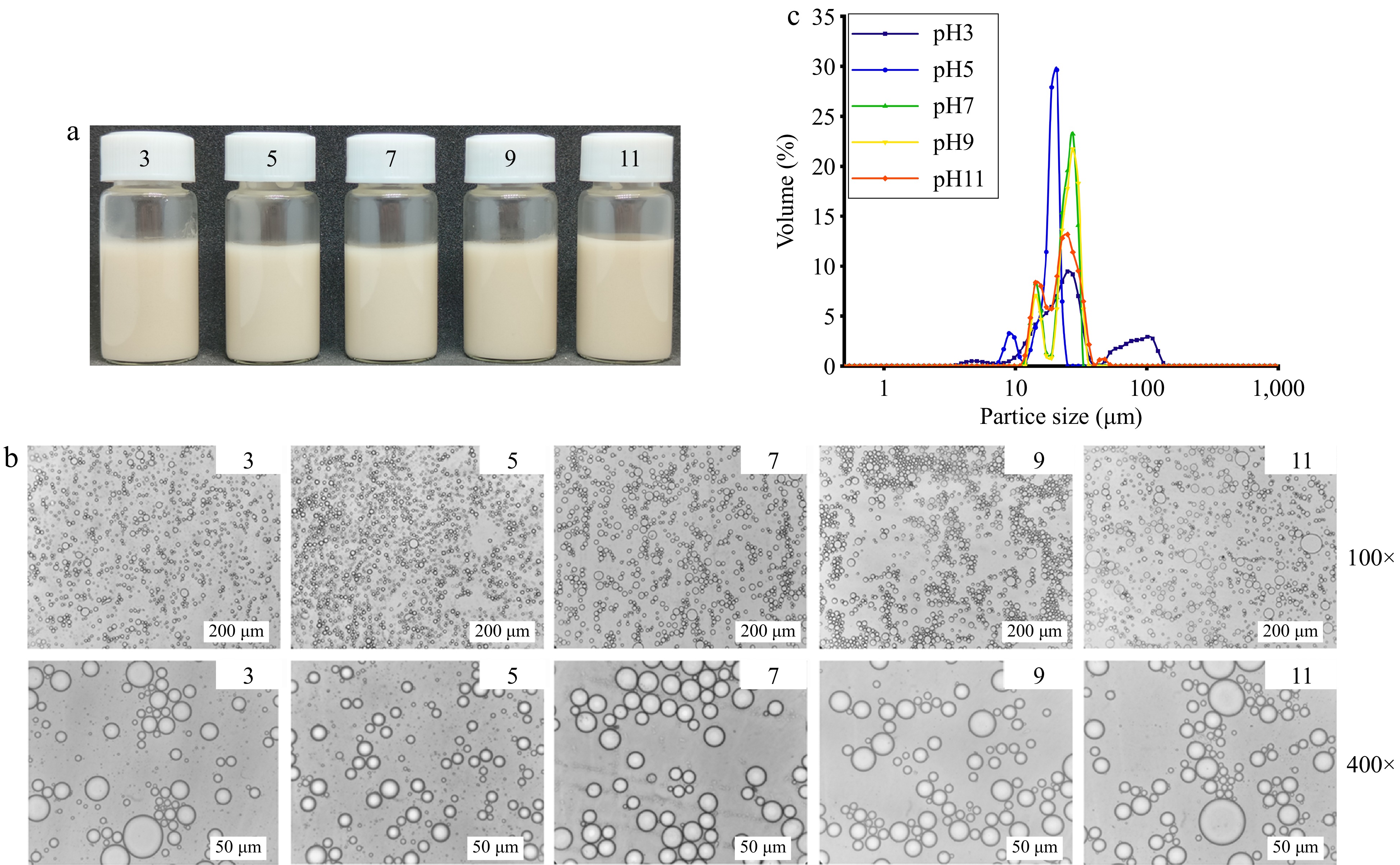
Figure 5.
Effect of pH on the stability of emulsions. (a) Static stability of emulsion. (b) Micromorphology of emulsion. (c) Particle size distribution of emulsion.
Effect of salt ion concentration on the stability of emulsions
-
Figure 6a showed the emulsions prepared at different salt ion concentrations (0−500 mM) for AAP-W concentration of 1%, φ = 0.5, pH = 7, all of which were stable. Figure 6b & c illustrated that the particle size of the emulsion enlarges with an increasing concentration of salt ions. This phenomenon is primarily attributed to the substantial influence of the solution's ionic strength on the emulsifier's performance. The charges and ions present on the surface of polysaccharide molecules engage in interactions, resulting in the formation of a charge shield that diminishes the emulsifying capability of the polysaccharide molecules. Nevertheless, no demulsification was detected, suggesting that the emulsion formulated with AAP-W possesses good stability against salt ions.
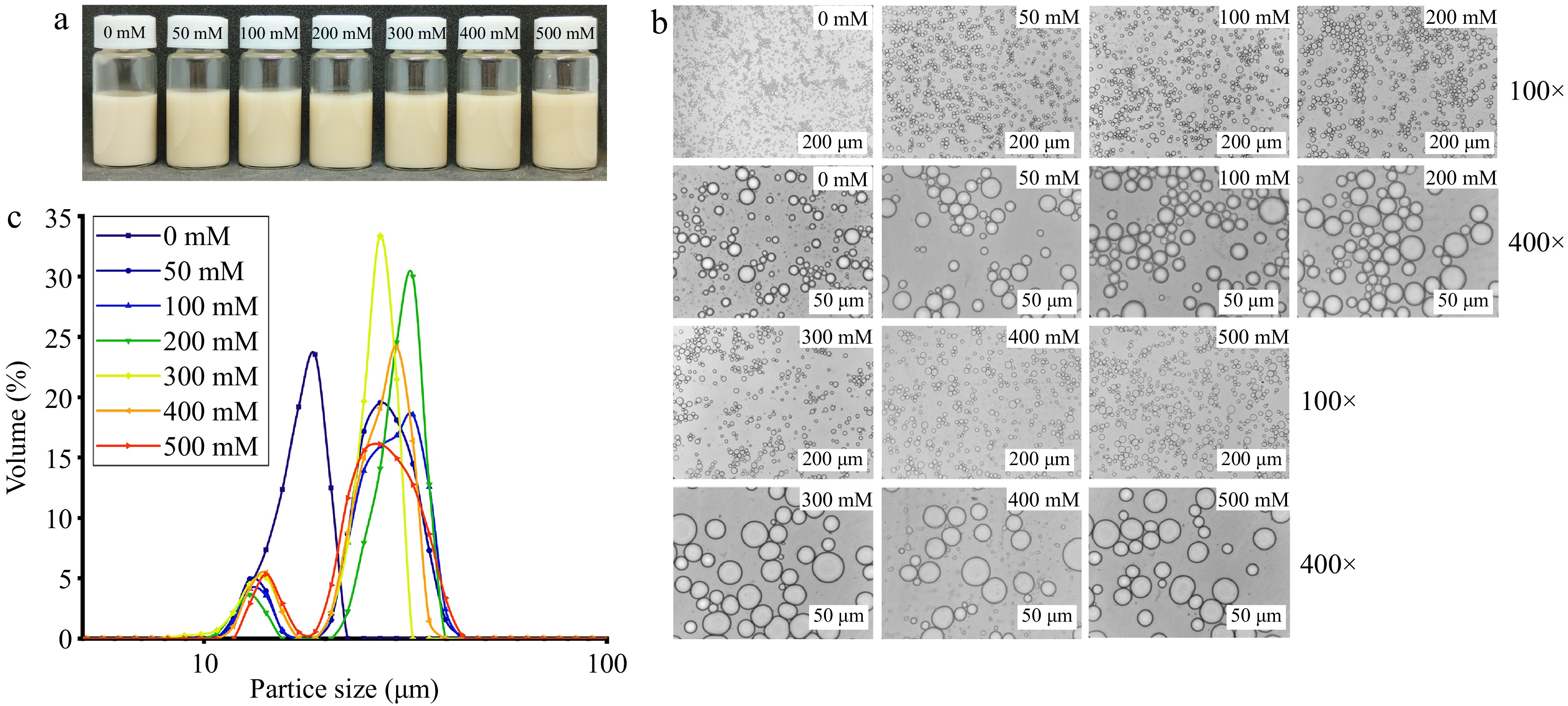
Figure 6.
Effect of salt ion concentration on the stability of emulsions. (a) Static stability of emulsion. (b) Micromorphology of emulsion. (c) Particle size distribution of emulsion.
Characterization of AAP-W emulsions loaded with β-carotene
Appearance of emulsion loaded with β-carotene
-
To improve the utilization efficiency of β-carotene, AAP-W emulsions loaded with β-carotene were prepared. Figure 7a showed that at the same concentration, β-carotene dissolved directly in oil was red, while loaded into the emulsion was orange-red without precipitation of aqueous and oil phases, indicating that the emulsion had good color stability and stationary stability.
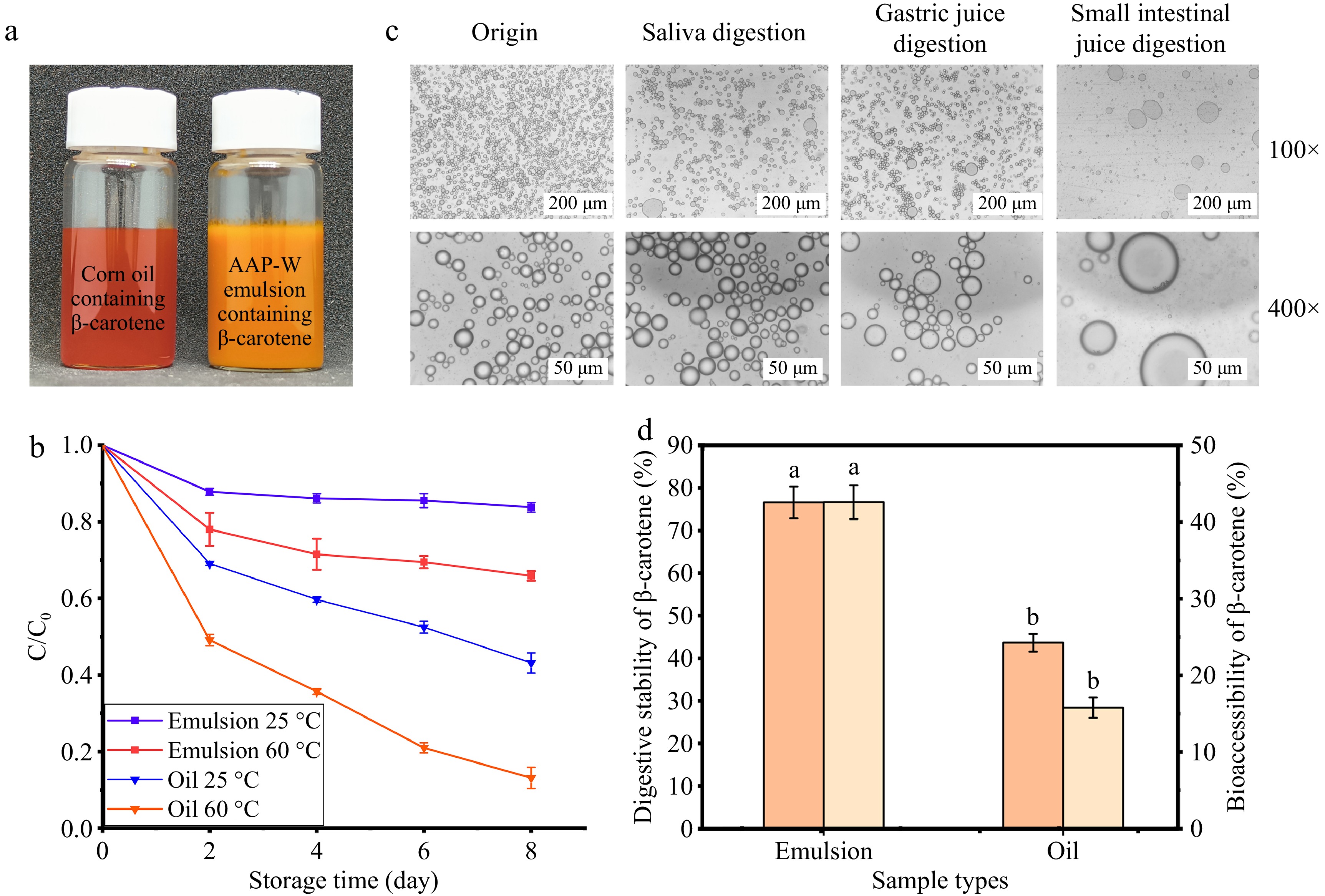
Figure 7.
Characterization of AAP-W emulsions loaded with β-carotene. (a) Load β-carotene corn oil and AAP-W emulsion. (b) Retention rate of β-carotene. (c) Changes of micromorphology of emulsion during in vitro digestion. (d) Digestive stability and bioavailability of β-carotene.
Variation of β-carotene retention in emulsions
-
Figure 7b shows the variation of retention of β-carotene loaded corn oil and emulsion at 25 and 60 °C, respectively. The retention of β-carotene decreases gradually with increasing storage time, regardless of the system and temperature. However, macromolecular polysaccharides were able to separate β-carotene from oxygen or free radical reactive substances in the surrounding environment, and they can also form an interface protection layer. Moreover, higher molecular weight (MW) leads to increased viscosity, which restricts the diffusion and transfer of the co-oxidant from the aqueous phase to the oil-water interface[61], thus slowing down the oxidation and degradation of β-carotene[58]. Similar results were obtained by Chen et al.[32].
In vitro digestion of loaded β-carotene emulsions
-
Figure 7c depicts the alterations in the micro-morphology of the droplets within the β-carotene-loaded emulsion throughout the digestion process. The droplet diameter of the original sample was the smallest and the most uniformly dispersed. Following digestion in the oral cavity and stomach, the emulsion still maintained a relatively intact droplet structure, but the density of the droplets underwent a significant decrease, and droplets with larger particle size began to appear. Both polysaccharide and lipid droplets are negatively charged in the simulated oral environment, and the electrostatic repulsion among droplets diminishes, resulting in droplet aggregation and an increase in particle size[62]. After digestion in the intestine, the droplet sizes of the emulsion was polarized between large droplets that had been formed through aggregation, and active ingredients that had been released from the droplets and were free from adsorption. Similar findings were found by Xu et al.[63].
Figure 7d showed a significant increase in bio-accessibility after β-carotene loading into the emulsion, which may be attributed to the faster digestion of the emulsion prepared by AAP-W. When the emulsion formulated with AAP-W reaches the small intestine, pancreatic lipase is rapidly adsorbed to the surface of the droplet via bile salts. This rapid adsorption enhances the lipase's ability to enter the hydrophobic lipid region, facilitating the rapid formation of mixed micelles that dissolve the droplets more quickly, thereby releasing β-carotene[64]. Similarly, the higher digestive stability of β-carotene under the loading of AAP-W emulsion may be because AAP-W is an anionic polysaccharide that could preferentially bind to metal ions in various digestive solutions and act as an antioxidant, thus inhibiting the oxidation of β-carotene and improving its stability.
-
Three Auricularia auricula polysaccharides (AAP-W, AAP-A, and AAP-AL) were obtained by hot water extraction, hot acid extraction, and hot alkaline extraction, and the results of their physicochemical properties and emulsification stability showed that AAP-W had better emulsification stability. Emulsions prepared with AAP-W as a natural emulsifier are well stabilized against harsh environments. Specifically, when the polysaccharide concentration rises to 1%, the emulsion can remain stable for a long time. The emulsions remained stable and did not emulsify when the oil phase volume fraction reached 0.6. The emulsions remained stable with only particle size changes over the range of pH (pH 5−11) and salt ion concentration (0−500 mM) measurements. In addition, the AAP-W emulsion system loaded with β-carotene was constructed to retard the oxidation and decomposition of β-carotene and to improve the bio-accessibility and digestive stability of β-carotene.
-
This paper does not contain clinical studies or data.
This work was supported by the National Natural Science Foundation of China (31901644), and the University Innovation Team of Shandong Province (2022KJ243).
-
The authors confirm contribution to the paper as follows: conceptualization, methodology, software, investigation, formal analysis, visualization: Lv X; writing - original draft: Lv X; writing - review & editing: Zhang D, Zhu X, Li D, Zhang C; supervision: Zhang C; resources: Zhang C. All authors reviewed the results and approved the final version of the manuscript.
-
This published article and associated supplementary information files contain all of the data generated or analyzed during this work.
-
The authors declare that they have no conflict of interest.
-
Authors contributed equally: Xiaqing Lv, Dexi Zhang
- Supplementary File 1 Supplementary methods.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Lv X, Zhang D, Zhu X, Li D, Zhang C. 2024. Emulsification stability of Auricularia auricula polysaccharides and its effect on steady-state properties of β-carotene embedding. Food Innovation and Advances 3(4): 360−371 doi: 10.48130/fia-0024-0034
Emulsification stability of Auricularia auricula polysaccharides and its effect on steady-state properties of β-carotene embedding
- Received: 19 August 2024
- Revised: 28 October 2024
- Accepted: 28 October 2024
- Published online: 26 November 2024
Abstract: The objective of the study was to examine the physicochemical properties and emulsification stability of three different Auricularia auricula polysaccharides (AAP) obtained through hot water extraction (AAP-W), hot acid extraction (AAP-A), and hot alkaline extraction (AAP-AL), respectively. The findings indicated that AAP-W exhibited superior emulsification stability compared to the other two polysaccharides. AAP-W was employed as a natural emulsifier for emulsion preparation, to examine the influence of varying polysaccharide concentrations and oil-water ratios on emulsion stability. Additionally, an investigation was conducted into the stability of the emulsions with respect to pH and salt ion concentration. The findings revealed that the most favorable polysaccharide concentration for the AAP-W emulsion was determined to be 1%, while the volume fraction of the oil phase was established at 0.5. It was also observed that the emulsion exhibited robust stability even in challenging conditions characterized by strong acidic (pH 3−5) or basic environments (pH 9−11), as well as high concentrations of salt ions (0−500 mM). Furthermore, the construction of an AAP-W emulsion system incorporating β-carotene was undertaken to enhance the preservation, bioavailability, and digestive stability of β-carotene, thereby expanding the potential applications of Auricularia auricula polysaccharides. This endeavor also presents a novel approach towards the advancement of novel functional food products.













