-
Ginger (Zingiber Officinale Roscoe) is a native plant of tropical Asia and Southeast Asia which is now cultivated worldwide[1]. In 2022, the total area of ginger cultivation worldwide was about 450,000 hectares, producing more than 4.87 million tons and China is the second-largest producing country[2].
Ginger is a fresh rhizome of perennial herbaceous plants in the ginger family and genus, which is a widely used medicinal and edible homologous plant. It contains a variety of chemical components, including gingerols, shogaols, curcumin compounds, terpenes, polysaccharides, amino acids, etc[3,4]. Due to these physiologically active ingredients, ginger has anti-inflammatory, anticancer, antioxidant, antiemetic, antibacterial, and other functions[5,6].
Curcumin compounds are a group of natural phenolic compounds existing in the rhizomes of some plants in the Zingiberaceae family[7], which gives food a unique flavor and yellow color. Curcumin has the largest content among them, along with demethoxycurcumin and bisdemethoxycurcumin[8,9]. Curcumin compounds possess an assortment of biological functions, such as anti-inflammatory, anti-cancer[10,11], and antioxidant[12] properties. In food, curcumin compounds are often used as food additives considering that they are common natural pigments and condiments[13]. In medicine, curcumin compounds have the characteristics of chemical prevention and treatment, and have high medicinal value[14].
At present, there are two main extraction methods for curcumin compounds, including traditional extraction methods and modern extraction methods[15−18]. Complicated specialized apparatus and equipment are usually needed for modern extraction methods like ultrasound-assisted extraction, pressured liquid extraction, and supercritical fluid extraction, although they may have better extraction effects. In comparison, traditional extraction methods like solvent extraction is more mature in technology[19]. The operation is relatively simple but requires more reagents. The main technique for the detection of curcumin components in ginger is high-performance liquid chromatography (HPLC)[20−22]. However, the detection was challenged by the low concentration and the concentration differences between curcumin compounds. High sensitivity and specificity in quantitative aspects could be obtained using multiple reaction monitoring (MRM) of tandem mass spectrometry (MS/MS) method, on the other hand, ultra-high-performance liquid chromatography (UHPLC) separates targeted substances effectively and reduce the disturbance of the complex matrix[23].
In the current study, an efficient and reliable analysis approach for curcumin, demethoxycurcumin and bisdemethoxycurcumin was established. Ginger specimens were obtained by the solid-liquid extraction method and then detected using UHPLC-MS/MS. The development of this detection approach can create a foundation for the mechanism study of curcumin compounds in ginger and benefit the deep utilization of curcumin components.
-
A Milli-Q Academic system (Millipore, Burlington, Massachusetts, USA) was employed to prepare ultra-pure water. Thermo Fisher Scientific Co., Ltd. (Beijing, China) provided LC/MS grade formic acid and ammonium formate, while Thermo Fisher Scientific Co., Ltd. (Waltham, Massachusetts, USA) provided LC/MS grade acetonitrile, methanol, ethanol, acetone, and ethyl acetate. Dimethyl sulfoxide (DMSO) (> 99.9%) was purchased from Bailing Wei Technology Co., Ltd. (Beijing, China). Curcumin (≥ 99.0%), demethoxycurcumin (≥ 99.8%), and bisdemethoxycurcumin (≥ 98.1%) standards were acquired from Alta Scientific Co., Ltd. (Tianjin, China).
Standard preparation
-
Considering the different polarities and sensitivity to light of the three curcumin compounds, methanol was utilized as a solvent to make a standard stock solution of curcumin and DMSO was used for demethoxycurcumin and bisdemethoxycurcumin then dissolved into a brown glass vial. Stock solution (50 mg∙mL−1) of each of the curcumin compounds was prepared. Then the mixed standard solution of 50 μg∙mL−1 was prepared using an aqueous solution containing 0.05% formic acid and acetonitrile containing 0.05% formic acid (1:1, v/v) and kept at −20 °C.
Sample preparation
-
Ginger samples used for method optimization and validation were purchased from local markets of Beijing, China. Before analysis, approximately 500 g of each ginger sample was washed, sliced, and dried in a freeze-drying machine for a duration of 120 h. The dried ginger samples were then ground using a grinder, passed through a 60-mesh sieve, and stored at −20 °C until analysis.
Ginger powders of 1.000 g (± 0.001 g) were precisely weighed and transported into a centrifuge tube with 30 mL methanol. The samples were then sonicated for 10 min at 20 kHz, oscillated at 250 rpm for 4 h, and centrifuged for 5 min at 10,000 rpm under 4 °C. The solution of supernatant was then filtered through a 0.22 μm microfilter into a glass vial for detection. Light was avoided during curcumin compounds extraction.
UHPLC-QQQ-MS analysis
-
Using a Thermo Scientific Ultimate 3000 system with a Waters X Select Peptide HSS T3 column (2.1 mm × 100 mm, 2.5 μm), the chromatographic separation was accomplished. A 0.05% formic acid aqueous solution served as mobile phase A, whereas a 0.05% formic acid-acetonitrile solution served as mobile phase B. The flow rate was set as 0.45 mL∙min−1, and the elution gradient was set as follows: 0−1.6 min, 50% B; 1.6−2.0 min, 50%−100% B; 2.0−5.0 min, 100% B; 5.1 min, 50% B and 5.1−8.0 min, 50% B. A temperature of 35 °C was maintained for the column, and 5 μL of injection volume was used.
Mass spectrometry analysis was conducted with Thermo Scientific TSQ Endura. Triple Quadrupole Mass Spectrometer coupled with an electrospray ion source under positive ion mode. Quantification was performed employing the MRM mode. The operation parameters were as follows: ion spray temperature, 320 °C; ion transfer tube temperature, 325 °C; ion spray voltage, 3,600 V; sheath gas, 30 psi; auxiliary gas, 10 psi; exhaust gas, 0 psi. The data were analyzed with Thermo Fisher XCalibur software for quantitative analysis.
Method validation
-
Method validation for the three curcumin compounds was carried out with sensitivity, linearity, accuracy, and precision. Limits of quantification (LOQ) and limits of detection (LOD) were used to assess the sensitivity. The LOD was calculated at the signal-to-noise ratio (S/N) of 3 and the LOQ was obtained at the S/N of 10. Using a solvent (methanol) standard calibration curve with concentration levels ranging from LOQ, linearity was evaluated. The slope approach was used to assess the matrix effect. The calculation formula of the slope method was 'Matrix effect = A/B × 100%', where A and B stand for the slopes of the matrix-matched calibration curve and the solvent standard calibration curve, respectively[24,25]. The accuracy of the approach was assessed by the spiking recovery with the spiking content which equals approximately 50%, 100%, and 200% of the background compound content in ginger samples. Relative standard deviations (RSD) were used to assess intra- and inter-day accuracy by injecting six replicates of the standard solution (200 μg∙L−1) within a day and over three consecutive days, respectively.
Statistical analysis
-
All sample results were collected by analysis of data processed through the Xcalibur program (Thermo Fisher Scientific, USA). Microsoft Office Excel 2019, Origin 2018, and SPSS Statistics 27.0 were used for data statistical analysis.
-
Triple quadrupole mass spectrometry (QQQ-MS) employing positive electrospray ionization mode was utilized to obtain the mass spectra of the three curcumin compounds. The protonated molecular ions [M + H]+ of m/z 369.2, 339.2, and 309.1 were the predominant precursor ions in their respective mass spectra (Table 1). Then, at an ideal collision energy, the ions underwent collision-induced dissociation.
Table 1. MS parameters of the three curcumin compounds.
Compound RT (min) Precursor ion (m/z) Product ion (m/z) Fragmentor (V) CE (V) Curcumin 2.75 369.2 285.1*/177.1 139 16/18 Demethoxycurcumin 2.45 339.2 255.1*/147.1 148 15/21 Bisdemethoxycurcumin 2.18 309.1 225.1*/147.1 128 13/21 * represents the quantitative ion. To develop an approach for the detection of curcumin compounds in ginger, two chromatographic columns were selected, namely Waters XBridge BEH C18 (2.1 mm × 150 mm, 3.5 μm) and Waters Xselect Peptide HSS T3 column (2.1 mm × 100 mm, 2.5 μm). The results showed that the HSS T3 column had better peak shape and higher resolution. This may be because HSS T3 is a fully porous silica filler with low bonding phase density, triple-bonded C18, and proprietary capping, which can operate in 100% aqueous mobile phase and retain polar and non-polar compounds, while BEH C18 is a fully porous hybrid filler with high bonding phase density and cannot retain polar compounds like curcumin compounds[26]. Based on the resolution of the peak shape and the response value, the Waters Xselect Peptide HSS T3 column was selected for the present study.
To attain the suitable separation efficiency for the targeted compounds, the chromatographic parameters were optimized. The water/methanol system and water/acetonitrile system were optimized as mobile phases. It was found that the three curcumin compounds could be separated in both mobile phase systems but showed a higher response in the water/acetonitrile system. It is known that adding acid or salt to the mobile phase can improve the shape of the chromatographic peaks and meanwhile, increase the analysis sensitivity. Generally, formic acid can stabilize the ion suppression of the eluents, while ammonium formate can improve the abundance of the protonated adduct and its product ion prominently[27]. Therefore, in this study, four different water/acetonitrile systems were tested, including water/acetonitrile, 0.05% (v/v) formic acid water/acetonitrile, 5 mmol∙L−1 ammonium formate water/acetonitrile, 0.05% (v/v) formic acid, and 5 mmmol∙L−1 ammonium formate water/acetonitrile. The results demonstrated that the separation of curcumin compounds was significantly improved after adding formic acid to the mobile phase, meanwhile, the analysis sensitivity was increased and the peak shape was also improved. This is because liquid chromatography conditions can affect the ionization efficiency of the target compound and ionization inhibitors can assist in chromatographic separation[28]. For this reason, the mobile phases for this research were chosen to be 0.05% (v/v) formic acid water and 0.05% (v/v) formic acid acetonitrile.
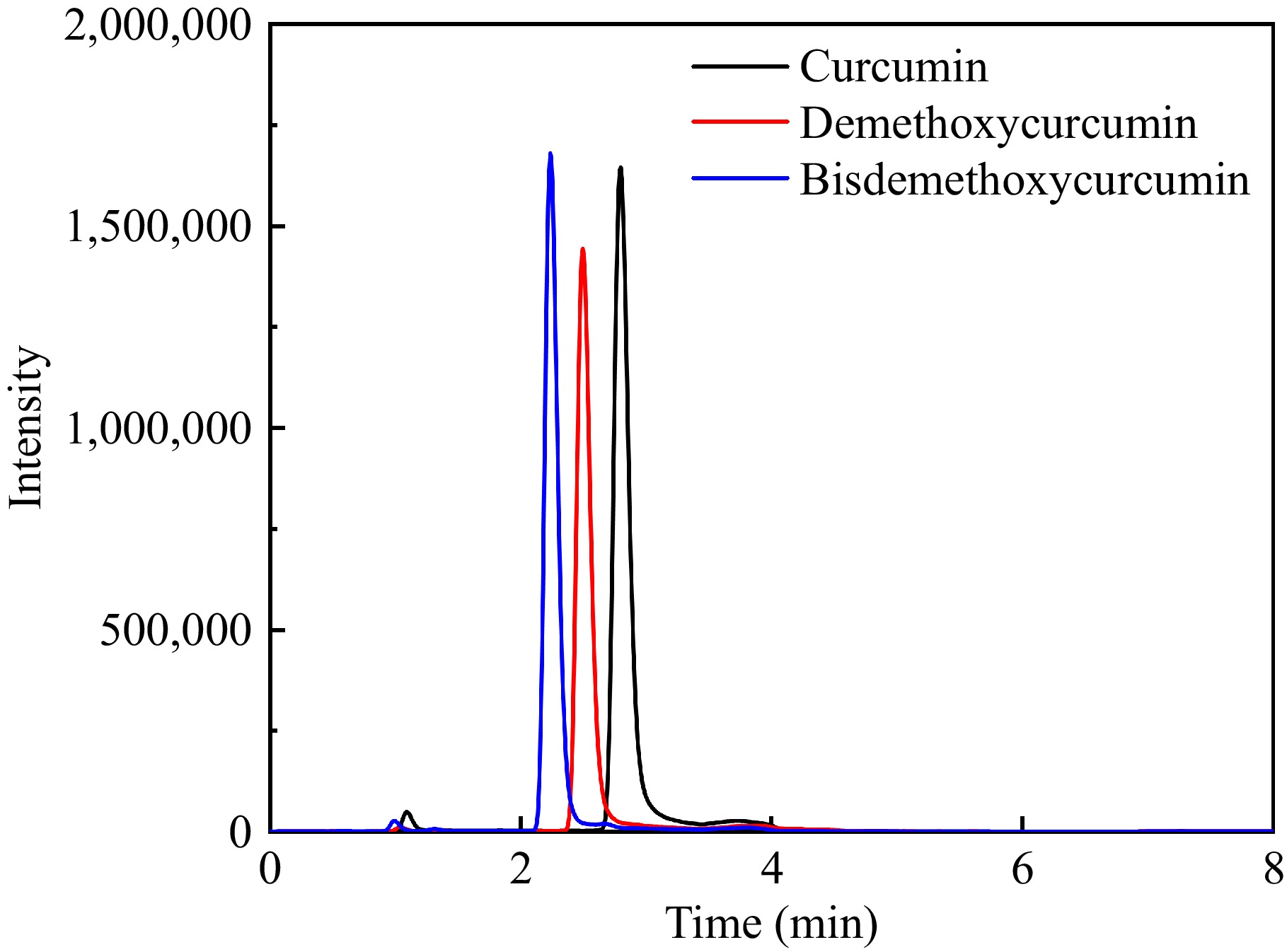
The three curcumin components were determined simultaneously for a total running time of 8 min. The absence of a co-elution peak within the retention time of curcumin, demethoxycurcumin, and bisdemethoxycurcumin indicated that this method has excellent selectivity. The extracted ion chromatogram of the three curcumin compounds acquired by UHPLC-MS/MS is shown in Fig. 1.
Optimization of extraction conditions
Extraction solvents
-
Bioactive ingredients have traditionally been extracted from food, and Chinese herbal medicine using solid-liquid extraction procedures.
Solid-liquid extraction techniques have traditionally been used for the extraction of bioactive ingredients from agricultural products, food and Chinese herbal medicine, including phenolic compounds, amino acids, flavonoids, terpenes, tannins, and alkaloids[29−32]. Considering the intricate nature of the ginger and the chemical and physical characteristics of the three curcumin compounds, the extraction solvent was optimized in the current study. Based on the literature, five different extraction solvents have been employed to extract curcumin compounds, including methanol, acetonitrile, ethanol, ethyl acetate, and acetone[12,33−35]. Therefore, these five solvents were optimized as the extraction solvent and the results of the extraction efficiency of curcumin, demethoxycurcumin, and bisdemethoxycurcumin are shown in Fig. 2a. Higher concentrations of the three curcumin compounds were obtained in the methanol extracts, and ethyl acetate exhibited the worst extraction efficiency. The results were consistent with those of Lee & Choung[36], who studied the extraction efficiency of curcumin compounds in 54 items of 16 commercial foods, and evaluated the extraction efficiency of solvents as follows: methanol > acetonitrile > ethyl acetate. Jorge-Montalvo et al. also found that solvents with relatively medium polarity (such as methanol with a value of 0.76) exhibited better extraction results of the antioxidant compounds like curcumin compounds in ginger[37]. Indeed, the solubility of compounds in solvents are consistent with the 'like dissolves like' principle. The extraction results were associated not only with solvent polarity but also with other parameters like molecular size, conformation, intermolecular hydrogen bonds, and solvent solute effects[38]. In addition to the extraction efficiency, the samples extracted with methanol had better chromatographic peak shape, which was narrower, sharper, and more symmetrical. For this reason, methanol was selected as the extraction solution in this study.
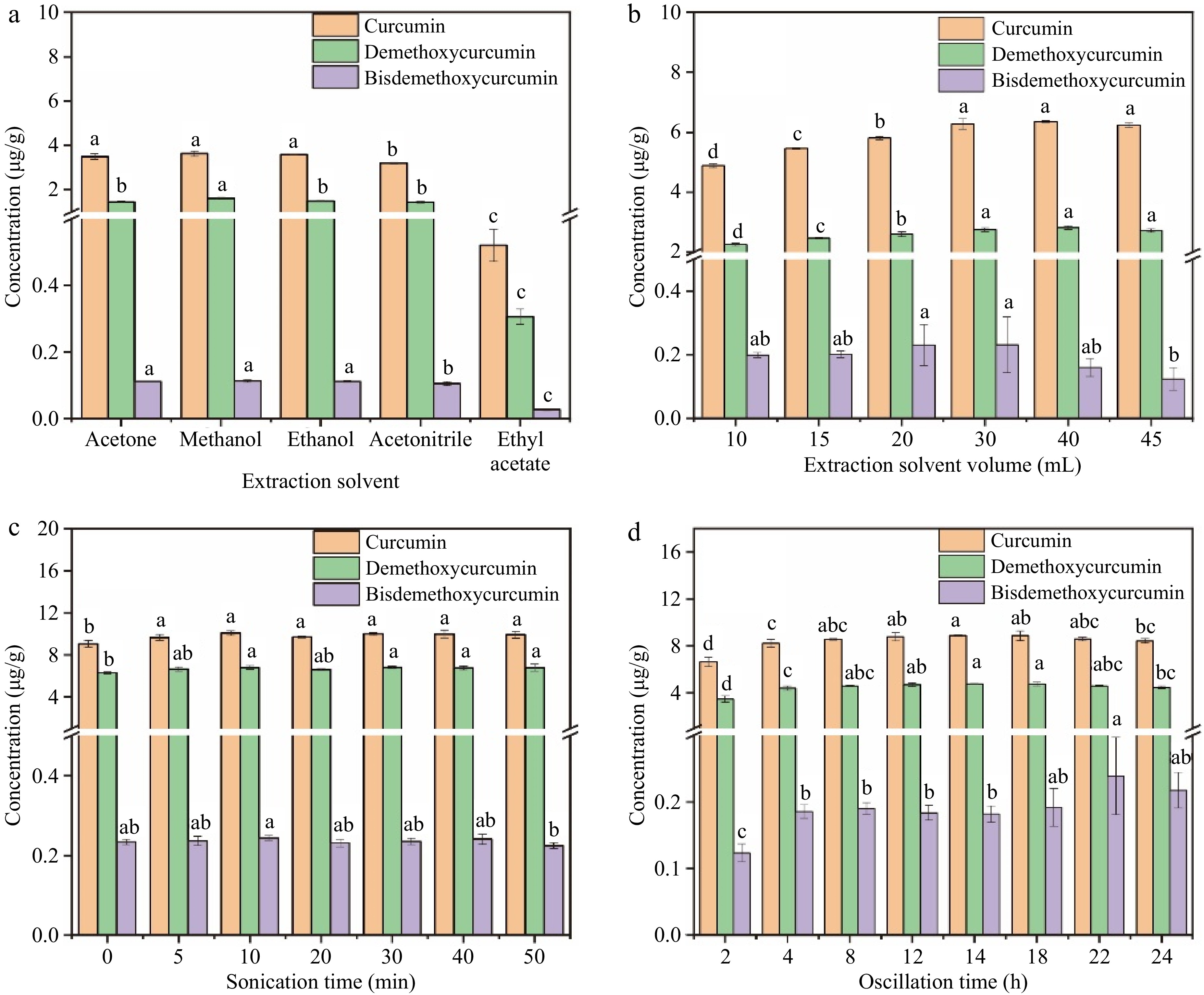
Figure 2.
Effects of extraction conditions on the extraction efficiency of the three curcumin compounds. (a) Extraction solvent, (b) extraction solvent volume, (c) sonication time, (d) oscillation time.
Extraction solvent volume
-
In the solid-liquid extraction process, the extraction solvent volume is essential to ensuring both an adequate extraction yield and a low cost. In the current study, different extraction solvent volumes of 10, 15, 20, 30, 40, and 45 mL were compared. As illustrated in Fig. 2b, the volume of methanol has significant impacts on the extraction results of curcumin compounds. With the increase of solvent volume from 10 mL to 30 mL, the concentration of the three curcumin compounds showed an upward trend. This was caused by the mass transfer principle, which meant that the driving force during the mass transfer process was the consistency gradient between the solid and the liquid. This gradient increased with increasing solvent-to-solid ratios, leading to improved extraction efficiency[39]. However, when the solvent amount was higher than 30 mL, there would not be enough liquid phase in relation to the dispersed phase to accomplish sufficient transfer that various equilibria may occur, leading to non-negligible resistance to mass transfer[40]. At the same time, with the increase in methanol volume, some unwanted lipids and other matrix components were also extracted. This explains why the extraction efficiency of the curcumin compounds did not elevate when the volume of methanol increased from 30 to 50 mL, and even showed a downward trend. Therefore, the optimal extraction solvent volume selected in this experiment was 30 mL.
Sonication time
-
Ultrasonication is an effective method for cell disruption in laboratory conditions[41]. To ensure the full extraction of curcumin compounds, the sonication time of the ginger powder and extraction solvent mixture was optimized. As shown in Fig. 2c, the extraction yield rose with the ultrasonic treatment time rising from 0 to 10 min (p < 0.05) and maintained at a stable level with the sonication time continuing to go up to 50 min. As is known, ultrasound has beneficial impacts in food analysis, which utilizes the cavitation effect, referring to a series of dynamic processes in which sound waves propagate in a liquid, causing periodic alternating changes in sound pressure, forming local compressed and expanded phases in the liquid[42]. Due to the nonlinear vibration of bubbles caused by cavitation and the explosion pressure generated when they burst, many physical or chemical effects could be generated along with cavitation, which induced a positive effect for extraction. However, ultrasonic cavitation could also generate extreme physical conditions such as high temperature, which was observed in the extraction with the increase of the sonication time and could cause the thermal degradation of the curcumin compounds[43,44]. The time cost is a significant reference for extraction and purification methods[45]. Therefore, considering the extraction efficiency and time cost comprehensively, a sonication time of 10 min was selected.
Oscillation time
-
Oscillation is another factor that affected the extraction efficiency of curcumin compounds. It is a common method for extracting targeted compounds, which mainly uses the mass transfer theory to produce the transfer of constituents in the system[46]. The oscillation time of 2–24 h was optimized in the current study and the results are illustrated in Fig. 2d. The extraction yield showed an upward trend with the increase of oscillation time from 2 to 8 h, and a significant increase were seen from 2 to 4 h (p < 0.05). After oscillating for more than 4 h, there was no significant differences in the extraction yield of curcumin compounds. Extraction time usually has positive effects on the yield of extracted compounds, and prolonging extraction time is beneficial for the extraction. Indeed, in the process of extraction, solid samples were wetted and dissolved by the solvents, and the release of components from the samples to the solvents takes a certain amount of time[47]. Previous studies have shown that the extraction yield increased with the prolongation of extraction time, but after a certain period of time, this increased rate decreases with the extension of time[48]. This may be the saturation of the targeted compounds in the extraction solvents, or even the degradation of some bioactive substances due to thermal exposure during the long-term extraction process[15]. Hence, 4 h was chosen as the optimal oscillation time.
Method validation
-
The LODs, LOQs, calibration linearity, spiking recovery, matrix effect, and precision were all examined to evaluate the performance of the current approach. As shown in Table 2, the LODs of curcumin, demethoxycurcumin, and bisdemethoxycurcumin were 0.003–0.006 mg∙kg−1, and the LOQs were 0.015 mg∙kg−1 (calculated by dry weight), indicating satisfactory detection sensitivity. The three curcumin compounds showed good linearities with coefficients of determination (R2) exceeding 0.9990. The matrix effects were between 94.6% and 98.8%, implying that there were negligible matrix interferences in ginger extracts and the solvent standard calibration curve can be used for method quantification. The method accuracy was evaluated using recovery at three spiking concentrations, which were approximately 50%, 100%, and 200% of the background compound content in ginger samples. The spiking levels were 4, 8, and 16 mg∙kg−1 for curcumin, 2.7, 5.4, and 10.8 mg∙kg−1 for demethoxycurcumin, and 0.15, 0.3, and 0.6 mg∙kg−1 for bisdemethoxycurcumin. The recovery of three curcumin compounds was 81.7%–100.0%, and the intra-day and inter-day precision expressed in RSD was lower than 5.4%. In conclusion, the approach of establishment is sensitive, accurate, and precise, indicating that the approach is reliable as well as appropriate for the quantification of curcumin components in ginger samples.
Table 2. Method validation results of the established methods.
Compound LOD (mg∙kg−1) LOQ (mg∙kg−1) Linear range (mg∙kg−1) R2 Matrix
effect (%)Recovery (%) ± SD Intra-day RSD (%) Inter-day RSD (%) Low Mid High Curcumin 0.006 0.015 0.015-30 0.9999 94.6 97.1 ± 10.7 90.4 ± 19.6 96.8 ± 5.6 5.3 1.8 Demethoxycurcumin 0.006 0.015 0.015-30 0.9990 98.8 100 ± 4.6 94.5 ± 6.1 95.2 ± 4.1 3.5 2.6 Bisdemethoxycurcumin 0.003 0.015 0.015-30 0.9995 96.1 85.0 ± 9.9 81.7 ± 10.5 83.8 ± 1.6 5.2 5.4 SD means standard deviation (n = 6); RSD means relative standard deviations (n = 6). Next, a comparison was made between the UHPLC-MS/MS method established in this study and the reported methods for the quantification of curcumin compounds in ginger samples. As shown in Table 3, the method validation assessment was entirely implemented only in this study and the current method exhibited the highest sensitivity with satisfactory linearity, matrix effect, accuracy, and precision.
Table 3. Comparison of the present study for the determination curcumin compound by different methods in ginger*.
Compound name Analysis methodb LOD LOQ R2 Matrix
effect (%)Recovery
(%)RSD (%) Ref. Curcumin HPLC-UV 0.028 ng∙mL−1 0.093 ng∙mL−1 0.9983 / > 98 0.94 and 1.07 (intra-day)
4.44 (inter-day)[20] Curcumin RRS 0.018 mg∙L−1 0.047 mg∙kg−1 1.000 / 97.7–103 2.0–2.6 (intra-day)
2.0–2.6 (inter-day)[49] DWO-VIS 0.013 mg∙L−1 0.33 mg∙kg−1 0.9999 96.3–104 2.3–2.6 (inter-day)
2.2–2.8 (intra-day)Curcumin UHPLC-IM-Q-TOF/MS 1.53 ng∙mL−1 5.09 ng∙mL−1 0.9910 / 93 1.17 (intra-day),
2.73 (inter-day)[23] Demethoxycurcumin 0.77 ng∙mL−1 2.57 ng∙mL−1 0.9922 106 1.91 (intra-day),
3.72 (inter-day)Bisdemethoxycurcumin 1.09 ng∙mL−1 3.63 ng∙mL−1 0.9906 85 2.85 (intra-day),
3.2 (inter-day)Curcumin UHPLC-MS/MS 0.006 mg∙kg−1 0.015 mg∙kg−1 0.9999 94.6 98.7–104.2 5.3 (intra-day),
1.8 (inter-day)Current
studyDemethoxycurcumin 0.006 mg∙kg−1 0.015 mg∙kg−1 0.9990 98.8 100.7–102.9 3.5 (intra-day),
2.6 (inter-day)Bisdemethoxycurcumin 0.003 mg∙kg−1 0.015 mg∙kg−1 0.9995 96.1 80.0–83.3 5.2 (intra-day),
5.4 (inter-day)'/', not provided. b. HPLC-UV, high-performance liquid chromatography; RRS, resonance Rayleigh scattering; DWO-VIS, dual wavelength visible absorption spectroscopy; UHPLC-IM-Q-TOF/MS, ultra-high-performance liquid chromatograph ion mobility quadrupole time-of-flight mass spectrometry; HPLC-MS/MS, ultra-high-performance liquid chromatography coupled with tandem mass spectrometry. Determination of ginger samples
-
To prove the applicability of the current method of solid-liquid extraction and UHPLC-MS/MS determination for the quantification of curcumin compounds in ginger, six ginger samples were randomly selected for quantitative analysis. The results are exhibited in Table 4. As shown in Table 4, the concentration of curcumin in all ginger samples was the highest, which was 0.575–10.143 mg∙kg−1, and the content of demethoxycurcumin was lower, with the value of 0.368–7.196 mg∙kg−1. The concentration of bisdemethoxycurcumin was the lowest of the three curcumin compounds, ranging from 0.019 to 0.281 mg∙kg−1. In addition, sample A had the lowest concentration of three curcumin compounds, while sample E had the highest concentration.
Table 4. Concentration of the three curcumin compounds in ginger samples.
Compound Concentration (mg∙kg−1)* Sample A Sample B Sample C Sample D Sample E Sample F Curcumin 0.575 ± 0.042 0.719 ± 0.008 5.228 ± 0.043 7.304 ± 0.095 10.143 ± 0.095 4.16 ± 0.073 Demethoxycurcumin 0.368 ± 0.029 0.511 ± 0.009 3.148 ± 0.042 4.589 ± 0.049 7.196 ± 0.081 3.05 ± 0.037 Bisdemethoxycurcumin 0.019 ± 0.001 0.019 ± 0.001 0.218 ± 0.009 0.276 ± 0.004 0.281 ± 0.005 0.191 ± 0.005 * The data were expressed as 'mean values ± standard deviation'. -
This study effectively established a quantitative approach using UHPLC-MS/MS for the simultaneous detection of curcumin, demethoxycurcumin, and bisdemethoxycurcumin in ginger samples. The method provides good sensitivity, negligible matrix effect, and good accuracy and precision, which indicates that the developed method was reliable and suitable for the quantitative detection of three curcumin compounds in ginger samples.
This work was supported by the Youth Innovation of CAAS (Y2023QC19), the China Agriculture Research System of MOF and MARA, the Young Elite Scientists Sponsorship Program by CAST (2023QNRC001), and the Agricultural Science and Technology Innovation Program of CAAS (CAAS-ASTIP-IQSTAP-2024).
-
The authors declare that they have no known conflict financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Jiang Y, Liu X, Zhao Y, Zhang J, Qiu J, et al. 2024. Quantitative analysis of curcumin compounds in ginger by ultra-high-performance liquid chromatography coupled with tandem mass spectrometry. Food Innovation and Advances 3(4): 353−359 doi: 10.48130/fia-0024-0033
Quantitative analysis of curcumin compounds in ginger by ultra-high-performance liquid chromatography coupled with tandem mass spectrometry
- Received: 30 July 2024
- Revised: 24 September 2024
- Accepted: 04 October 2024
- Published online: 30 October 2024
Abstract: Curcumin compounds are important bioactive compounds in ginger, yet their analysis is limited by their low concentrations. In the current research, a highly sensitive and reliable approach for simultaneous quantitative detection of three curcumin compounds in ginger samples was established using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS). The extraction solvent, volume of extraction solvent, sonication time, and oscillation time were optimized by a single factor experiment. The method validation results showed that the regression coefficients were higher than 0.9990, and the linearity was satisfactory. Matrix effects were negligible with the values of 94.6%–98.8%. The recovery at three spiking levels was between 81.7% and 100.0%, and the precision was less than 5.4%. The approach could be used to determine the curcumin components in ginger samples since the results demonstrate that it is easy to use, practicable, repeatable, and accurate.
-
Key words:
- Curcumin /
- Demethoxycurcumin /
- Bisdemethoxycurcumin /
- UHPLC-MS/MS /
- Ginger