-
Fermentation is a long-standing food-processing technology through which humans have invented alcoholic beverages that hold an important position in politics, economy, and culture[1]. In Western practices, the fermentation of wine and beer relies on yeast, which requires the prior hydrolysis of starch in the ingredients due to the absence of its own starch-digesting enzymes[2]. However, Eastern techniques have ingeniously circumvented this enzymatic limitation by leveraging the synergistic action of diverse microbial cultures, harnessing a range of enzymes produced by indigenous microorganisms. Therefore, mixed fermentation agents are considered indispensable substances in alcohol fermentation in many regions of Asia, and this mixed fermentation agent is what we now know as 'Jiuqu'[3]. Jiuqu is a time-honored saccharification and fermentation medium used to achieve the decomposition of starch from multiple grains as substrates and the fermentation of alcohol. As early as the Northern Wei Dynasty in China, Jia Sixie recorded the differences and preparation methods of nine types of Jiuqu in 'Qi Min Yao Shu'. Fermentation starters used in liquor production can be generally classified into Daqu, Xiaoqu, and Fuqu based on their raw materials, functions, and production processes[3]. Daqu primarily utilizes barley, wheat, and peas as raw materials and contains a highly complex microbial community, which results in the production of a wide variety of aromatic compounds. In contrast, Xiaoqu mainly uses rice bran or rice flour as the primary substrate and is characterized by a shorter fermentation period, higher yield, lower starter dosage, simpler production process, and lower cost as compared to Daqu[4]. Fuqu, primarily made from wheat bran, is a fermentation starter optimized through microbiological techniques. It reconstructs a simplified microbial community and exhibits strong saccharification capabilities, high efficiency, and low production costs[5]. Among these various types of fermentation starters, Daqu holds a central role as the primary starter in the production of Chinese Baijiu[6].
Daqu serves as the fundamental structure in the Baijiu production process, with its variety and excellence directly influencing the final product's taste and quality[7]. From the perspective of flavor characteristics, Daqu can be categorized into four types: (1) Jiang-flavor Daqu, such as Maotai, is characterized by a soy sauce flavor, rich body, and lasting aroma[8]; (2) Strong-flavor Daqu, such as Luzhou Laojiao, is characterized by a rich aroma, soft taste, and endless aftertaste[9]; (3) Light-flavor Daqu, such as Fenjiu, is characterized by pure flavor, is sweet and mellow, and refreshing aftertaste[10]; (4) Miscellaneous-flavor Daqu, such as Baiyunbian Baijiu, is characterized by sensory characteristics between Jiang-flavor and strong-flavor Baijiu[11]. The four types of Daqu have different characteristic aroma compounds. The distinctive flavors of Baijiu and the variety of its aromatic volatile compounds can be attributed to the diverse microbial populations within Daqu. These variations in the microbial makeup result in a range of enzymatic profiles within Daqu, which directly affect the synthesis of its flavor compounds[12].
The quality of fermented foods is strongly influenced by the metabolism of microbial communities, as well as factors like moisture, temperature, and other environmental conditions. However, even with the rapid development of technology today, it is difficult to fully control these environmental factors. Therefore, microbial inoculation technology has been developed to enhance the quality of fermented foods[13]. By inoculating designated fermenting agents, it is possible to improve and guide the fermentation process to achieve the enhancement of targeted product characteristics[14]. At present, inoculation technology has been widely used in the field of fermented foods, such as the inoculation of Lactobacillus in sausages, which has improved the texture and flavor[15].
The inoculation of Saccharomyces cerevisiae and Acetobacter malorum during the fermentation process of strawberries ensures the correct and rapid acetification for strawberry vinegar[16]. The introduction of Zygosaccharomyces rouxii, Candida versatilis, and Tetragenococcus halophilus into the fermentation process of fish sauce effectively prevents the development of off-flavors[17]. Similarly, for Daqu, which is usually produced in a semi-controlled manner based on experience in family or small workshops, microbial inoculation technology has become a key technology to improve its quality[18].
To date, research on Daqu has mainly concentrated on the dynamics of microbial communities, flavor attributes, and biochemical properties. However, the functional enzyme system in Daqu is often overlooked. This article starts from the perspective of Daqu's flavor profile, first summarizing the types and production techniques of Daqu. It then examines the pathways for producing the primary flavor substances in Daqu and explores the roles and contributions of various functional enzymes in the development of Daqu's flavor. Finally, the discussion turns to the current techniques for improving Daqu flavor through microbial inoculation, including the advantages, shortcomings, and bottlenecks of microbial inoculation. The findings can provide references for the efficient production of premium Baijiu Daqu.
-
Throughout the Daqu production process, temperature serves as a pivotal factor that influences the assembly of microbial communities, which in turn is a key criterion for classifying Daqu types. The categorization of Daqu is determined by the maximum fermentation temperature recorded throughout its manufacturing cycle[12]. Based on the peak hatching temperature attained through the natural metabolism of microorganisms, the following types of Daqu can be produced[19]: (1) Low-temperature Daqu, reaching peak temperatures of 40 to 50 °C during incubation, usually producing Qing-flavor Daqu, represented by Fenjiu and Erguotou; (2) Medium-temperature Daqu, reaching peak temperatures of 50 to 60 °C during incubation, usually producing Strong-flavor Daqu, represented by Wuliangye; (3) High-temperature Daqu, reaching peak temperatures of 60 to 65 °C during incubation, usually producing Jiang-flavor Daqu, represented by Maotai.
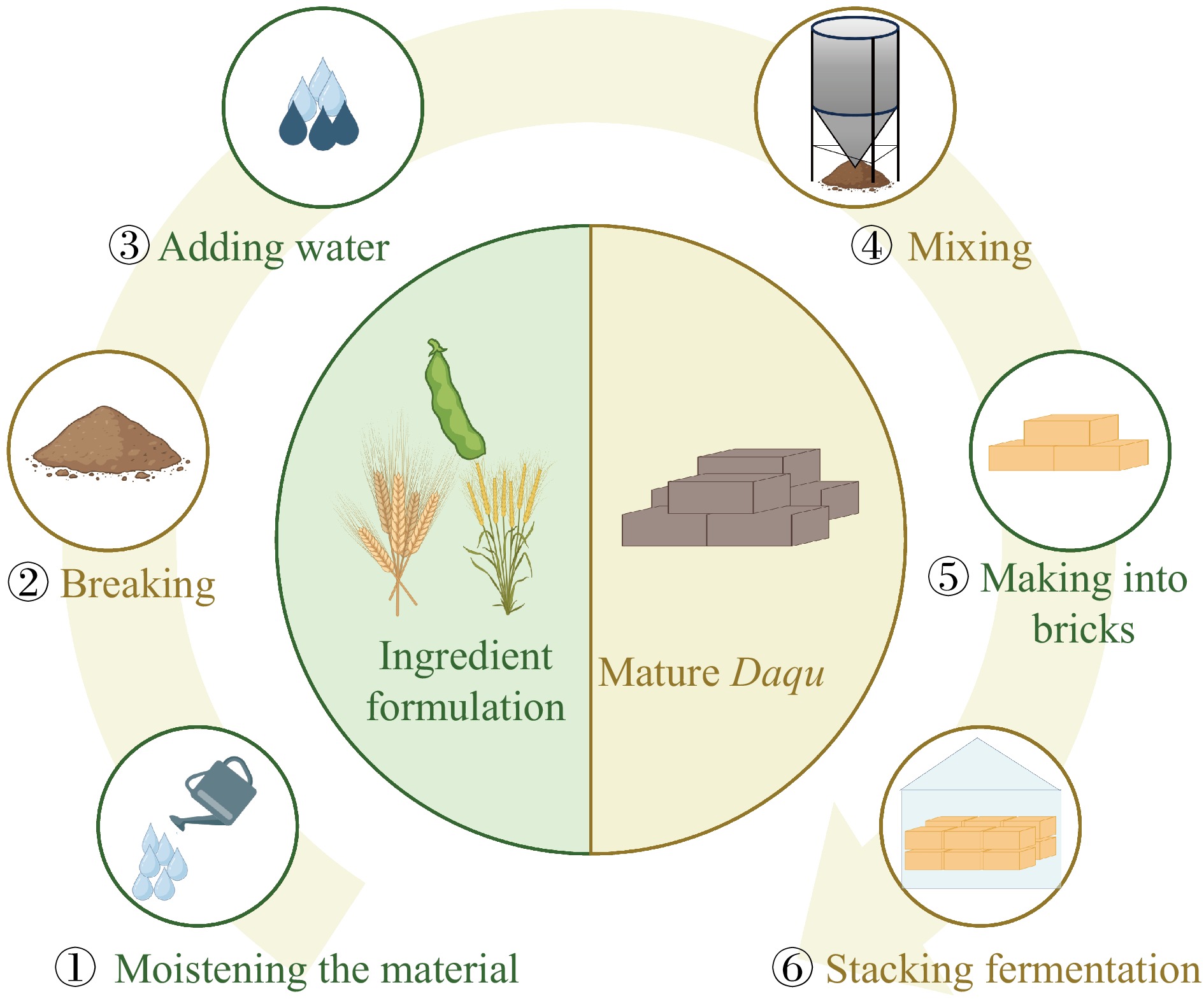
The production process of Daqu has a long history in China. Daqu is usually made from wheat, barley, and peas through solid-state fermentation, produced through the metabolic activities of natural microbial communities. Six main steps, namely batching, grinding, mixing, forming into blocks, incubation, and storage[12], are commonly involved in the production of Daqu as follows. (1) Batching: When wheat, barley, and peas are used as raw materials for making Daqu, the ratio is usually 5:4:1, 6:3:1, or 7:2:1. (2) Grinding: The degree of raw material grinding affects the quality of Daqu. If it is too fine, it will be too sticky, with small pores inside the Daqu block, poor air permeability, slow microbial growth, and difficulty in dissipating moisture and heat. If it is too coarse, it will be less sticky, with large pores inside the Daqu block, easy to dissipate moisture and heat, prone to premature drying and cracking, poor microbial growth, and other problems. (3) Mixing: The purpose of mixing is to increase the moisture content in the raw materials, promoting the growth and reproduction of microorganisms. The usual water-to-mixing ratio for wheat, barley, and pea mixed Daqu is 40%~45%. (4) Forming into blocks: The general size of the brick-shaped Daqu block is (30~33) cm × (18~21) cm × (6~7) cm. (5) Incubation: The prepared Daqu is stored in the Daqu room, and fermented in stacks at different temperatures according to the type of Daqu. (6) Storage: Once the fermentation process is concluded, Daqu undergoes a period of storage lasting 8 to 10 d with the aim of reducing its moisture level to 15% and allowing the temperature to stabilize at ambient levels. Following this, it is placed in a warehouse for a further six-month storage period[20,21]. Of course, the Daqu production process in different regions will also vary according to the actual local conditions. Figure 1 shows the main production steps of traditional Daqu.
The fermentation of Daqu is a long-term spontaneous process, traditionally relying heavily on manual labor, which results in high labor intensity and production costs, but low efficiency. Due to the open nature of manual Daqu preparation, the quality is significantly influenced by operational variability, making it difficult to maintain consistency. To address those challenges, Daqu production is gradually transitioning towards mechanization and automation. Mechanized production has already been applied in steps such as raw material pretreatment and brick shaping. For the traditional manual Daqu-stepping process, some mechanical equipment has already been designed to imitate human foot movements. By utilizing a multi-stage pneumatic cylinder system to step on the Daqu in phases, manual labor has been replaced, and both the quality stability and production efficiency of high-temperature Daqu have been significantly improved. With the advent of Industry 4.0 and the rapid development of artificial intelligence (AI), improvement using AI of Daqu production is gradually becoming feasible. Xiao & Li[22] attempted to integrate ZigBee and WiFi networks using a ZigBee protocol stack to enable real-time monitoring of environmental temperature and humidity and remote data transmission. Thus, an electronic sensing system combined with machine learning has the potential to identify and predict key Daqu parameters and quality. The strength of intelligent technologies lies in their integration of computer science with large datasets, offering a promising solution to the challenges in the brewing industry. These technologies are highly efficient in detection, classification, and prediction, particularly excelling in handling nonlinear relationships, temporal and spatial variability, and multifactorial influences[23]. By applying intelligent technology, it becomes possible to effectively predict fermentation states, regulate the production processes, assess product quality, classify brewing grades, and forecast production outcomes[24].
-
The flavor of Daqu is a key indicator of its quality, and variations in flavor will directly result in different types of Baijiu. The main representative aroma compounds of Jiang-flavor Baijiu have been identified to be tetramethylpyrazine while that of strong-flavor Baijiu is ethyl hexanoate[25]. The principal flavor constituents of miscellaneous-flavor Baijiu include caproic acid, ethyl caproate, heptanone, and butyric acid[11]. At present, the study of flavor compounds mainly relies on technologies such as gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), and gas chromatography-flame ionization detection (GC-FID)[26].
Zhou et al.[27] conducted a comprehensive analysis and documented a diverse array of 456 volatile compounds in Daqu before 2018. This chemically rich profile encompassesed a spectrum of compounds, such as esters, alcohols, acids, and aldehydes, which are critical for the characteristic flavors of Baijiu. Specifically, the study identified four types of esters, 62 alcohols, 41 acids, and 43 aldehydes, along with other significant contributors to the flavor profile, including 36 pyrazines, 34 ketones, and 34 aliphatic compounds. Additionally, the research highlighted the presence of 25 hydrocarbons, 25 other heterocyclic compounds, 18 furans, and 16 nitrogen-containing compounds, among others. The study also examined the presence of phenolic, ether, sulfur, aromatic hydrocarbon, terpene, lactone, and carbonyl compounds, with counts of 14, 11, 10, 9, 8, 7, and 3 types respectively. Moreover, from the perspective of Daqu types, 255 flavor substances were detected in high-temperature Daqu, 328 flavor substances in medium-temperature Daqu, and 140 flavor substances in low-temperature Daqu. The synthesis of these flavor compounds involves various functional enzymes[28]. Ethyl hexanoate is the main flavor compound in strong-aroma Baijiu, and its synthesis pathway primarily involves the esterification of hexanoic acid and ethanol, catalyzed by relevant esterification enzymes (such as 3-hydroxybutyryl-CoA dehydrogenase, EC: 1.1.1.157)[29]. Glucose undergoes glycolysis to produce pyruvate, which is then converted to acetyl-CoA. Under the catalysis of alcohol acyltransferase, acetyl-CoA reacts with ethanol to form ethyl acetate[30]. Pyruvate dehydrogenase (EC: 1.2.4.1) catalyzes the conversion of pyruvate to acetyl-CoA, making it crucial for the synthesis of ethyl esters and other fatty acid esters. Lactic acid, an important flavor compound in Baijiu, plays a key role in reducing the harshness of ethanol. Lactate dehydrogenase reversibly catalyzes the conversion of pyruvate to lactic acid[31].Figure 2 shows the main pathways for the production of the main aroma substances in Daqu. The aroma substances in Daqu mainly come from the decomposition of biological macromolecules (starch, protein, lipids, etc.) under the action of microorganisms and enzymes[32]. Firstly, carbohydrate and energy metabolism are the foundation of microbial growth and also the basis and premise for the production of most flavor substances. The process primarily involves carbohydrate metabolism, glycolytic conversion, pyruvate transformation, and the operation of the citric acid cycle[25]. The intermediate substances produced in these processes are crucial for the production of ethanol and flavor substances. In the TCA cycle, acetyl-CoA is a key precursor for the production of the majority of acetate esters found in Daqu[33]. In addition, acetyl-CoA also plays a crucial role in the metabolism of various flavor intermediates (such as acetoin, fatty acids, etc.)[34]. Owing to the swift advancement in omics-based techniques, our comprehension of the catalytic roles of enzymes within the tricarboxylic acid (TCA) cycle continues to expand. Yi et al.[35] demonstrated the presence of key enzymes involved in the tricarboxylic acid (TCA) cycle, specifically isocitrate dehydrogenase (EC 1.1.1.41), malate dehydrogenase (EC 1.1.1.37), and succinate dehydrogenase (EC 1.3.5.1). These enzymes have been identified in species including Aspergillus oryzae, Thermoascus stipitatus, Aspergillus clavatus, members of the Trichocomaceae family, the phylum Firmicutes, and Emericella nidulans.
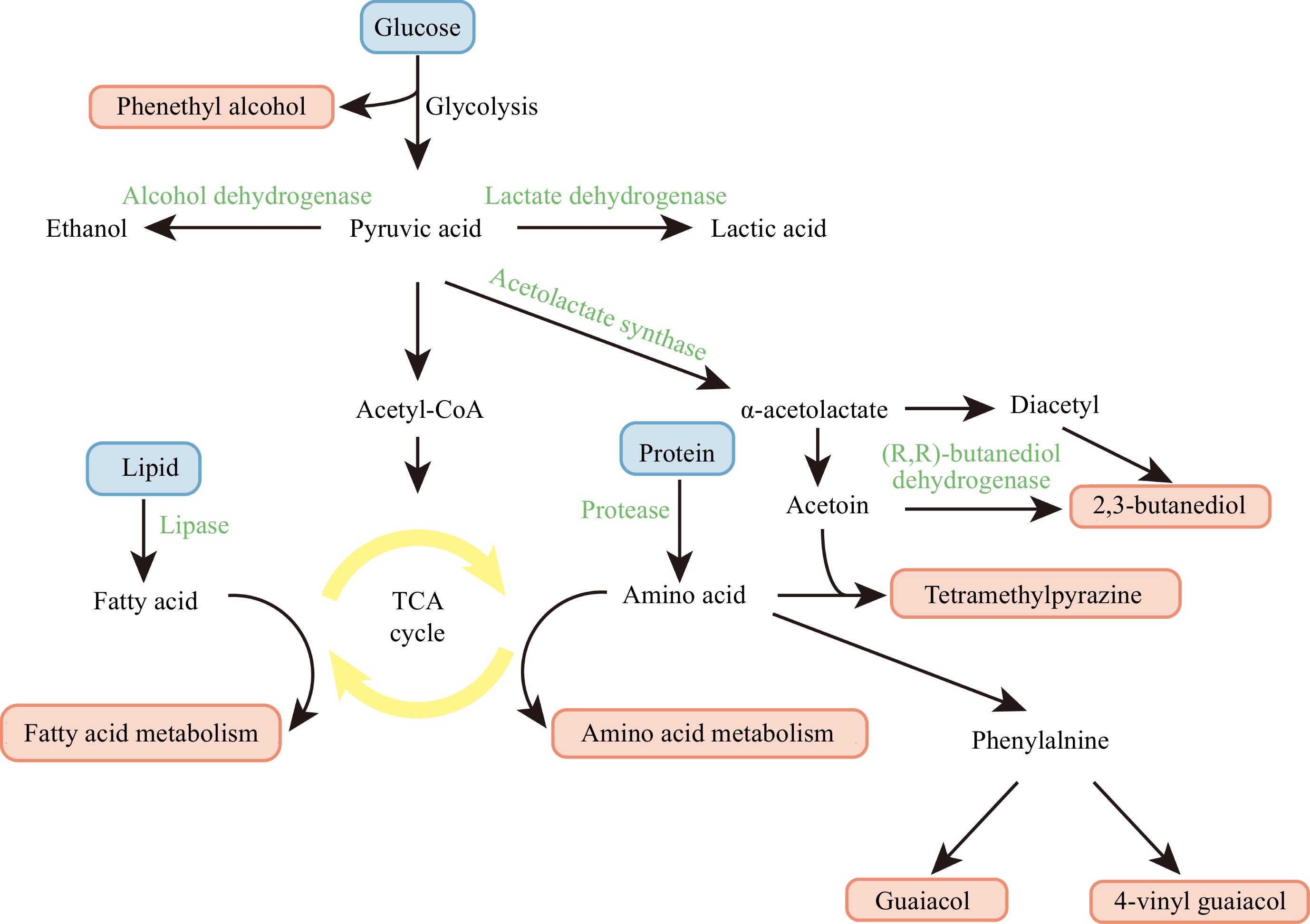
Figure 2.
The production pathways of the main aroma substances in Daqu. Green represents the enzymes involved in each stage, while red indicates the aromatic compounds produced.
Secondly, proteins are also an important source of flavor substances. glutamic acid (Glu), Valine (Val), leucine (Leu), isoleucine (Ile), phenylalanine (Phe), alanine (Ala), tyrosine (Tyr), arginine (Arg), and glutamine (D-Gln) from protein degradation can serve as intermediates for Daqu flavor substances[31]. In addition, pyrazine substances are also an important type of flavor substances, which are mainly produced through the Maillard reaction between sugars and amino acid residues[36]. These alkylpyrazines usually have nutty, baked, and roasted notes, and their formation is related to the acetoin and ammonium (NH3) pathway[37]. Among, tetramethylpyrazine is the representative aroma compound of Jiang-flavor Daqu. In addition, compounds such as guaiacol, tetramethylguaiacol, and benzyl alcohol can provide spicy and clove-like flavors to the qu. Yi et al.[35] discovered 11 metabolic pathways linked to the synthesis of aromatic compounds via transcriptomic analysis, encompassing pathways for the breakdown of various aromatic substrates including aminobenzoate, benzoate, fluorobenzoate, chlorobenzoate, ethylbenzene, naphthalene, biphenyl, styrene, dimethylbenzene, polycyclic aromatic hydrocarbons, and toluene.
Additionally, the metabolism of esters significantly contributes to the development of Daqu's distinctive flavor profile. The main ester substances in Daqu include ethyl hexanoate, ethyl acetate, ethyl butyrate, ethyl valerate, and ethyl heptanoate, which usually impart fruity, floral, and honey-like flavors to Baijiu[27]. The alcohols and acids produced by ester metabolism are also important components of Daqu flavor. The main acids in Daqu include acetic acid, butyric acid, and hexanoic acid, among which hexanoic acid gives Baijiu a pungent and sour taste. The main alcohol substances in Daqu include n-butanol, isoamyl alcohol, and n-pentanol, among which n-butanol and n-hexanol have an apple-like flavor[38].
Currently, Daqu manufacturing takes place in an environment that is not fully controlled, with environmental parameters like solar radiation, moisture levels, and thermal conditions exerting substantial influence on Daqu's microbiota and enzymatic profiles, thereby potentially driving the formation of unforeseen flavor compounds[39]. The metabolism of Streptomyces may produce geosmin, which can lead to earthy and moldy flavors in Baijiu[40]. Liu et al.[41] reported the detection of pathogens such as small copper bacteria and fecal enterococci in the finished Daqu and processing environment, particularly in the fermentation room and storage room. The metabolic by-products of these bacteria not only affect the flavor of Baijiu but also people's health. In the entire Daqu production process, enzymes produced by various microbial metabolisms influence the flavor and quality of Daqu. Hence, elucidating the enzymatic pathways involved in the generation of Daqu's flavor substances is a significant area of inquiry for advancing the flavor enhancement of Daqu in subsequent studies.
-
The enzymatic profile within Daqu is instrumental in the fermentation dynamics, with the diversity and concentration of these enzymes are pivotal to the final quality of the product. The main functional enzymes in Daqu include amylase, protease, cellulase, hemicellulase, tannin enzyme, pectinase, phytase, lipase, esterase, lacquer enzyme, etc.[42]. Different types of enzymes play different roles and functions during fermentation. Amylase primarily facilitates the liquefaction and saccharification stages of Daqu fermentation, whereas protease and esterase significantly influences the synthesis of flavor compounds, including ethyl hexanoate[43,44]. The following is an elaboration on different functional enzymes that involved in the production of Daqu (Table 1).
Table 1. The sources of enzymes with different functions in Daqu.
Types of enzymes Microbial sources Refs Type Genera Enzymes related to carbohydrates metabolism,
EC 3.2.1.XAmylase Bacteria Bacillus; Kroppenstedtia; Leuconostoc; Staphylococcus; Thermoactinomyces [57,62,65,71,83] Fungi Aspergillus; Byssochlamys; Penicillium; Rhizomucor; Saccharomycopsis; Thermoascus [50,62,84] Glucoamylase Bacteria Leuconostoc; Weissella [85,86] Fungi Aspergillus; Byssochlamys; Monascus; Rhizomucor; Rhizopus [84,87−90] Pectinase Bacteria Enterococcus [85,86] Fungi Eurotium; Streptomyces [91,92] Glucosidase Fungi Aspergillus; Lichtheimia; Saccharomycopsis; Paecilomyces; Thermoascus [50,89,93−95] Xylanase Fungi Aspergillus [88] Cellulase Fungi Penicillium [50] Hemicellulase Fungi Thermomyce [62] Enzymes related to proteins metabolism,
EC 3.4.X.XNeutral protease Bacteria Bacillus; Lactobacillus; Staphylococcus [54,57,70,71,96,97] Fungi Aspergillus; Eurotium; Lichtheimia; Mucor; Penicillium; Rhizomucor; Rhizopus [44,50,62,87,93,
94,97]Acid proteases Fungi Saccharomycopsis [52,98,99] Fibrinolytic enzyme Fungi Rhizopus [97] Fibrinogenase Bacteria Bacillus [70] Enzymes related to esters metabolism,
EC 3.1.1.XLipase Bacteria Bacillus [54,71,100] Fungi Eurotium; Rhizomucor; Rhizopus [87,89,97] Esterase Bacteria Bacillus; Leuconostoc; Staphylococcus; Weissella [65,70,71,85,97] Fungi Issatchenkia; Lichtheimia; Mucor; Monascus; Penicillium; Streptomyces; Zygosaccharomyces [44,50,57,66,78,
89,94,95,101]Other enzymes Tannase Bacteria Bacillus [65] Fungi Aspergillus; Penicillium [50,93] Alkaline phosphatase Fungi Streptomyces [57] Phosphate hydrolase Fungi Streptomyces [57] Glycosyltransferase Fungi Rhizopus [87,97] Enzymes related to carbohydrate metabolism
-
Saccharification is the process where polysaccharides (starch, cellulose, hemicellulose, etc.) in grains are hydrolyzed into fermentable sugars by enzymes, marking the beginning of Baijiu fermentation. Daqu plays an important role in this step by providing a variety of enzymes involved in saccharification (Fig. 3).
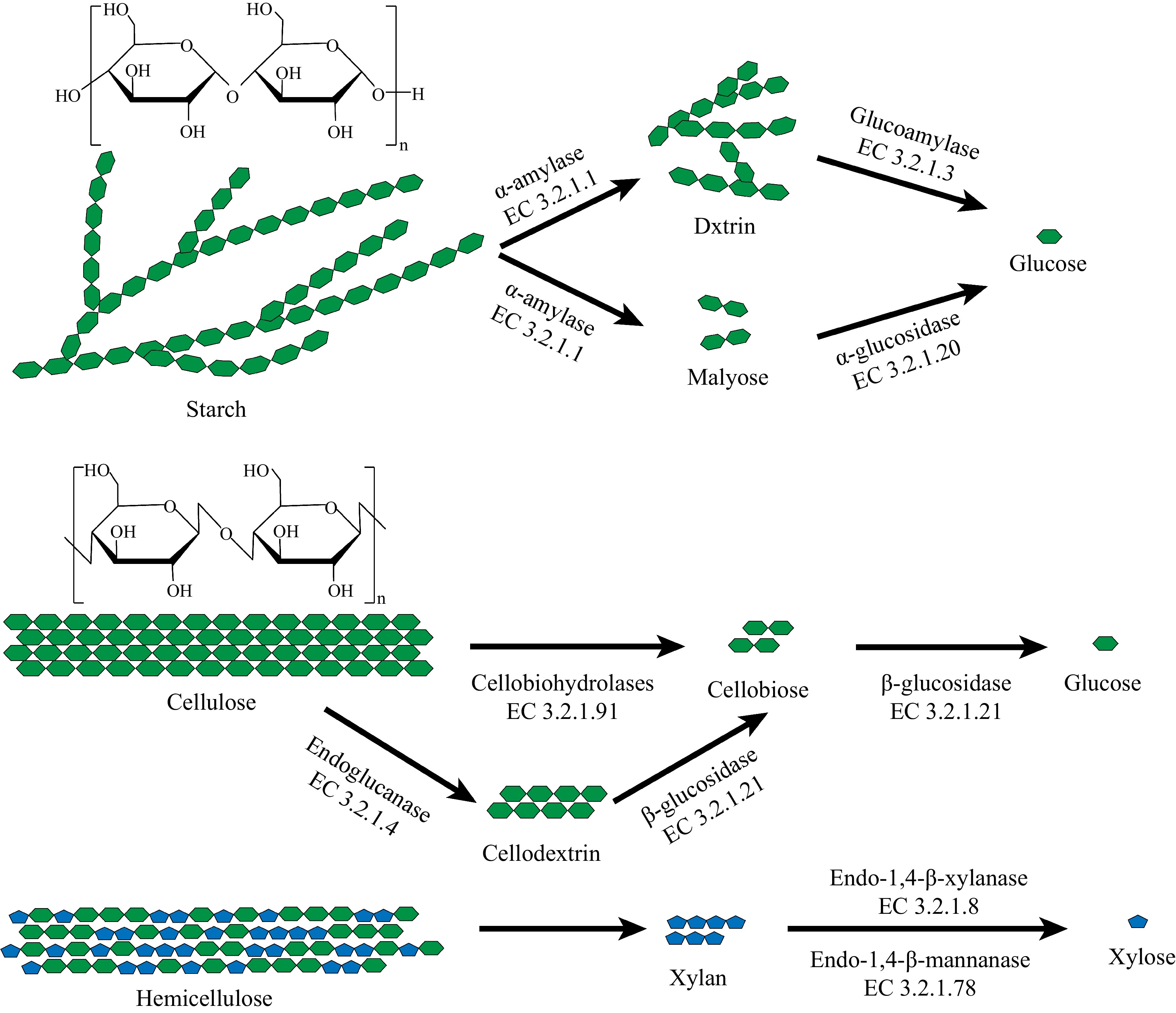
Figure 3.
The main pathways of saccharification metabolism in Daqu. Green represents hexoses, and blue represents pentoses.
Carbohydrates in grains (such as rice and sorghum) account for 73.6%−76.6%[45]. The saccharification process is mainly dominated by starch metabolism. Starch can be hydrolyzed by hydrolytic enzymes into dextrin, maltose, and glucose to be utilized by other microorganisms. The main enzymes involved in this process are α-amylase (EC 3.2.1.1), α-glucosidase (EC 3.2.1.3), etc.[46]. α-amylase and α-glucosidase can act on the α-1,4-glucosidic bonds inside the starch and the α-1,4-glucosidic bonds at the non-reducing end of the starch, respectively, thus hydrolyzing starch into small molecular sugars[47−49]. Therefore, they exert a significant influence within the Baijiu industry and are also known as liquefaction power (the ability of liquefying enzymes in Daqu to convert starch from a macromolecular state into a low-molecular-weight state) and saccharification power (the ability of saccharifying amylases in Daqu to hydrolyze starch into glucose), respectively. It is widely believed that molds are primarily responsible for breaking down large molecular substances (such as starch) in the raw materials during the initial fermentation stage, providing the essential enzymatic activity for the entire fermentation system[50]. The discovery of a strain of Aspergillus niger with an amylase activity as high as 6,800 U/mL suggests that it could serve as an excellent source of functional strains for γ-amylase and as a co-culture strain for the reinforcement of Daqu[49]. Du et al.[51] reported that Rhizopus oryzae, Aspergillus flavus, and Aspergillus oryzae, which are sources for Daqu production, can produce a large amount of saccharification enzymes and hydrolytic enzymes. Wang et al.[52] reported that Saccharomycopsis fibuligera, an advantageous yeast species in low-temperature Daqu can produce amylase and β-glucosidase, which degraded starch into dextrin, maltose, and glucose, providing nutritional supply for brewing yeast and many other microorganisms involved in Baijiu fermentation. High-temperature Daqu's genes for α-glucosidase, α-amylase, and saccharification enzymes belong to the genera Rhizopus, Aspergillus, and Rhizomucor, which are considered excellent functional strains due to their saccharifying characteristics[53]. Wang et al.[54] purified a glucoamylase from Aspergillus oryzae in Daqu, which exhibits broad substrate specificity, good thermal stability and pH stability, demonstrating excellent potential for industrial application in bioethanol production. Wang et al.[50] reported that the production capacity of saccharification amylase from mold strains in high-temperature Daqu, especially from the genera Aspergillus and Rhizopus was significantly higher than that of other enzymes. Rhizomucor pusillus is an important source of saccharification enzymes in fermented foods such as Jiang-flavor Daqu and light-flavor Daqu[55]. Interestingly, amylase plays a significant role in light-flavor Daqu and is positively correlated with the levels of glycerol, malic acid, and succinic acid. This is crucial for enhancing its biochemical properties, producing unique metabolic products, and creating a distinct flavor[44]. In addition to molds, some bacteria are also contributors to amylase. The genera Thermoactinomyces vulgaris and Thermoactinomyces sacchari have a strong ability to produce amylase, which gives them a strong capacity to catalyze the hydrolysis of starch[56,57]. Bacillus amyloliquefaciens and Bacillus subtilis are aroma-producing strains capable of secreting α-amylase, enabling the rapid fermentation of sugars in raw materials[58].
In addition to starch, cellulose, and hemicellulose in grains can also be hydrolyzed into sugars by cellulase and hemicellulase for use by other microorganisms[59]. Cellulase and hemicellulase are complex enzymes, mainly including endo-glucanase (EC 3.2.1.4), β-glucosidase (EC 3.2.1.21), β-xylosidase (EC 3.2.1.37), and xylobiose hydrolase (EC 3.2.1.91)[60]. The gene for β-glucosidase in cellulase comes from the genera Aspergillus and Mucor[61]. Thermoascus is a major contributor to the cellulase system[62]. Gou et al.[63] reported that Thermomyces lanuginosus, a thermophilic fungus capable of surviving at temperatures above 60 °C, is an efficient producer of xylanase. Identifying the sources of these key functional enzymes lays the groundwork for future targeted synthesis of these enzymes for the enhancement of Daqu.
Enzymes related to protein metabolism
-
Proteolytic enzymes, a diverse group of biocatalysts, target peptide bonds within proteins, facilitating their cleavage into smaller peptide fragments and individual amino acids. Proteolytic enzymes catalyze the breakdown of proteins present in the substrate into smaller peptides and amino acids during the fermentation phase of Baijiu production. Metabolites act as a nitrogen supply for the fermentation processes of yeasts and lactic acid bacteria, thereby facilitating the proliferation of these microbes throughout the fermentation period[64]. In addition, some of the protein degradation products, such as amino acids, are themselves flavor substances, while others can serve as precursors for aroma substances. Protease activity can affect the types and amounts of alcohols and organic acids in Baijiu, leading to changes in the quantity and quality of the final esters, resulting in different flavor types of Daqu. Therefore, proteases are indispensable enzymes in the Baijiu production process[65]. It is particularly noteworthy that proteases play a pivotal role in the development of the distinctive Jiang flavor profile in high-temperature Daqu, a critical component in the fermentation process. The enzymatic activity of proteases catalyzes the transformation of flavor precursors, significantly influencing the taste and aromatic characteristics of the end product[44]. Aspergillus flavus is recognized for its significant contribution to the production of acidic proteases in Daqu[50]. Monascus purpureus is capable of producing both acidic and neutral proteases that catalyze the esterification of acids and ethanol. This enzymatic activity is crucial for the transformation of precursor compounds into esters, which are fundamental to the distinctive aroma of Baijiu[66]. Wang et al.[67] successfully broadened the application scope of high-temperature actinomycetes by screening a strain from the Jiang-flavor Daqu that exhibited protease activity as high as 214.99 U. Thermoascus and Rasamsoni have been found to be positively correlated with high protease activity and amino nitrogen content in white Daqu[68]. Bacillus, as one of the most representative and important bacteria in Daqu, also has a strong ability to produce proteases[65,69−71]. For example, Bacillus licheniformis shows strong protease activity and can produce aromatic compounds, pyrazines, organic acids, etc.[71−74]. Liu et al.[65] reported that the protease strains purified from the strong-flavor Daqu all come from the genus Bacillus. Feng et al.[75] screened a strain of Bacillus pumilus that produces neutral protease from the light-flavor low-temperature Daqu, with a protease activity as high as 202.7 U. In addition to Bacillus, lactic acid bacteria are also major contributors to proteases. The products obtained through protease hydrolysis can participate in the Maillard reaction to produce aromatic substances[76].
Enzymes related to ester metabolism
-
The lipases present in Daqu are capable of breaking down fats from the raw materials into fatty acids, glycerol, as well as monoglycerides, among others. These compounds offer a source of energy essential for the microbial proliferation and reproduction[37]. In addition, some of the breakdown products of fats (such as fatty acids, monoglycerides, etc.) are of great significance for the taste, aroma, and other qualities of Baijiu. Yan et al.[77] reported that the addition of lipase to yellow water increased the concentration of flavor esters in Baijiu by 32 times.
Esterases are a class of enzymes that can hydrolyze or synthesize ester bonds in fats. Through the esterification process between carboxylic acids and alcohols, they are capable of generating aromatic esters that enhance the synthesis of flavor compounds in Daqu, consequently influencing the quality of the resulting Baijiu[44]. Esterases are believed to contribute similarly to the formation of the strong-flavors and Jiang-flavors in high-temperature Daqu. Candida have long been considered highly correlated with the esterification power (the capacity of esterases in Daqu to catalyze the synthesis of esters from free organic acids and ethanol) of Daqu[76]. A strain of Issatchenkia orientalis, recognized as an aromatic yeast that produces esterases, has been discovered to participate in the production of ethyl acetate in Baijiu. This yeast strain also exhibits good salt and ethanol tolerance[78]. Bacillus cereus is recognized for its capability to synthesize ethyl acetate, a key aromatic constituent in Chinese Baijiu, via the enzymatic activity of esterases. This production is significant for the distinctive flavor profile of the spirit[79]. It is worth noting that the relative abundance of lactic acid bacteria in Daqu is not as high as that of other dominant bacteria[80]. However, their contribution is significant in shaping the intrinsic qualities of Daqu. Wang et al.[81] identified a diverse group of lactic acid bacteria genera in high-temperature Daqu, encompassing Lactobacillus, Weissella, Pediococcus, Enterococcus, Leuconostoc, and Streptococcus. They are capable of generating a range of enzymatic catalysts, including esterases, which facilitate their involvement in the Maillard process to generate aromatic compounds and also yield substantial quantities of lactate esters' precursors, specifically lactic acid. Lactate esters serve as key precursors in the production of Baijiu, contributing to the enhancement of its mellow and sweet characteristics[80,82].
Other types of enzymes
-
With the development of genomics and proteomics, more functional enzymes have been discovered in Daqu. Isocitrate dehydrogenase, malate dehydrogenase, and succinate dehydrogenase from Aspergillus oryzae, Aspergillus clavatus, and Aspergillus terreus were involved in the formation of Baijiu flavor[35]. Acetyl-CoA not only participated in the biosynthesis of acetate esters but also in the metabolism of flavor intermediates such as fatty acids, ketones, and acetoin[59]. Streptomyces species, which are airborne isolates from the Baijiu production facility, exhibit enzymatic capabilities, including esterase, alkaline phosphatase, and phosphatase hydrolase production. These enzymatic activities significantly contribute to the synthesis of flavor compounds and their precursors in Jiang-flavor Baijiu[57]. The hydrolysis of stored glycogen or oligosaccharides catalyzed by phosphorylases was conducive to ethanol production and various phosphorylases have been widely used in industry due to their economic role in glycosyl transferase reactions[54].
Currently, research on the quality of Daqu primarily focus on microbial communities, while the role of functional enzyme systems in Daqu, which serve as a crucial link between Baijiu flavor and the structure of the microbial community is not yet well understood. Therefore, efforts should be focused on the identification of key enzymes and their contribution to the quality of Daqu. An integrated multi-omics strategy could be applied to analyze the origin of key functional enzymes during Daqu fermentation, as well as enzymatic properties and substrate specificity etc. Subsequently, the dynamics of biological enzymes in Daqu processing could be mimicked with recombinant enzymes, which would help in understanding the mechanisms that are involved in the quality formation of Daqu and standardizing of Daqu production.
-
In the Baijiu brewing process, the solid-state fermentation undergoes a series of complex and dynamic transformations. These encompass the proliferation of microbial populations, the utilization of nutrients and oxygen, the synthesis and accumulation of metabolic byproducts, fluctuations in temperature, and the evaporation of moisture, all of which contribute to the intricate biochemistry of the fermentation environment[102]. Li et al.[103] discovered that environmental factors exert significant differential impacts on microbial communities during various fermentation stages. In the mesophilic fermentation stage, moisture content and acidity are the primary determinants of microbial composition, whereas in the cooling and maturation stages, core temperature, and pH become the critical factors influencing microbial communities. Furthermore, they noted that bacterial communities might exhibit greater sensitivity to environmental changes than fungal communities. In the natural fermentation of Daqu, the heterogeneity of environmental factors lead to the diversification of Daqu colors, including white, yellow, and black. These different-colored Daqu samples show significant variations in microbial composition, physicochemical properties, enzymatic characteristics, and potential metabolic functions[68]. Fermentation temperature is regarded as a key controlling factor in the production of Daqu, as it influences the growth and death of microbes, thereby determining the succession dynamics of microbial communities[104]. The optimal growth temperatures for different microbial species vary, with molds and yeasts thriving at approximately 30 °C. Therefore, during high-temperature fermentation processes, the growth of these microbes is inhibited. In contrast, bacteria generally tolerate a broader temperature range, with some species capable of surviving between 40 and 60 °C. As a result, the influence of fermentation temperature on bacterial growth is less pronounced as compared to that on fungi[105]. These factors all affect the quality of fermentation to a certain extent[106]. It is currently very difficult to completely control all environmental factors during the fermentation process[86]. Thus, inoculated fermentation has become an important method to enhance the quality of fermentation products. Similarly, in the field of Baijiu brewing, achieving targeted improvement of Baijiu flavor and quality through inoculated fermentation has become a hot topic of current research.
The research on microbial inoculation of Daqu is still mainly focused on the development of excellent functional strains[13]. Previous research has consistently highlighted Bacillus as a quintessential and significant bacterial genus across diverse Daqu types, underscoring its pivotal role in these fermentation substrates[65,70,71]. The diversity and concentration of Bacillus species are decisive factors for the quality of Daqu, consequently dictating the sensory attributes and aromatic profiles of Baijiu[78]. Bacillus has been established as a principal functional bacterium in the production of Baijiu, possessing a robust capacity to secrete a range of degradative enzymes, including protease, amylase, and saccharifying enzymes. The exceptional capacity for enzyme synthesis in Bacillus confers upon it the ability to generate a diverse array of flavor compounds throughout the Jiang-flavor Baijiu fermentation. As a result, Bacillus has emerged as a favored candidate for inoculated fermentation practices.
Inoculation with Bacillus licheniformis has increased the amylase activity and the content of tetramethylpyrazine in Daqu, significantly enriching the aromatic compounds in Daqu, successfully regulating the metabolism of the microbial community within Daqu, and enhancing its flavor[106,107]. Daqu inoculated with Bacillus velezensis and Bacillus subtilis exhibited significantly higher liquefaction, saccharification, and esterification powers compared to regular Daqu. Furthermore, the inoculation of Daqu has led to a marked increase in the levels of esters, pyrazines, alcohols, and other volatiles, thereby significantly enhancing the Daqu's overall quality[70,108]. In addition to inoculating Bacillus, some prokaryotes have also become good co-inoculants. Chen et al.[109] inoculated Lactobacillus brevis, which contributed to an enhanced production of flavor compounds during fermentation, consequently reducing the Baijiu fermentation cycle and achieving improvements in fermentation efficiency and quality.
In addition to prokaryotes, many eukaryotes are also excellent functional strains. The flavor profile and fermentation efficiency of Baijiu are significantly influenced by the activities of wine yeast and ester-producing yeast, which generate key enzymes like lipases, esterases, and acyltransferases[13]. Fan et al.[18] inoculated Wickerhamomyces anomalus and Saccharomyces cerevisiae, and the mixed fermentation of the two yeasts increased the content of flavor compounds in Baijiu particularly ethyl acetate. Li et al.[110] reported that by inoculating Saccharomyces cerevisiae Y7#09 or Clavispora lusitaniae YX3307, the content of ethyl hexanoate in Baijiu was increased, resulting in the enhanced flavor of Baijiu. In addition to yeasts, molds also make a great difference in Baijiu fermentation. It is generally believed that molds are the main contributors to the degradation of large molecular substances (such as starch) in the raw materials during the initial stage of fermentation and provide the basic enzymatic power for the entire fermentation system[106]. Zhu et al.[111] reported that by inoculating Saccharomycopsis fibuligera and Rhizopus microsporus, more pleasant esters, such as isoamyl acetate, and octanoic acid ethyl ester, were produced in the wine medicine, causing the yellow wine to have a richer fruit aroma.
Compared with the inoculation of a single strain, the inoculation of mixed strains is a more popular choice. The co-inoculation of Bacillus, Pediococcus, Wickerhamomyces, and Saccharomycopsis into Daqu has successfully enhanced the dynamic stability of the microbial community during the solid-state fermentation process of Daqu[112]. The inoculation combination of Bacillus, Saccharomycopsis, and Absidia has had a positive impact on the amylase activity, key volatile compounds, and microbial community diversity in Daqu[113]. The mixed inoculation of Clostridium butyricum, Rummeliibacillus suwonensis, and Issatchenkia orientalis successfully increased the content of butyl acetate and hexanoic acid ethyl ester in strong-flavor Baijiu[114]. Inoculating Aspergillus niger and Saccharomyces cerevisiae altered the microbial community, increased ethanol production, and the content of aromatic compounds in the fermented grains, thereby enhancing the quality of Baijiu[115].
However, the strategy of enhancing fermentation in Daqu production using mixed microbial cultures still faces several challenges. Firstly, Daqu production is highly dependent on environmental conditions, and the inoculation of mixed microbial communities may lead to fluctuations in its physicochemical properties, thereby affecting the stability of its functional expression and aroma formation. Secondly, there is a lack of unified standards for beneficial microorganisms in Daqu and Baijiu production, with significant variation across different regions worldwide. This highlights the need for evidence-based approaches to improve quality and underscores the importance of establishing standardized protocols to ensure industry-wide consistency. Additionally, the use of mixed functional microorganisms to enhance Daqu production, along with the dynamic changes in microbial community functions during maturation, significantly influences the composition of volatile compounds and enzyme activities. Therefore, before adopting advanced biotechnological methods to tailor Daqu microbial communities for the improvement of Baijiu quality, it is essential to adhere to regulatory standards and conduct comprehensive safety assessments.
-
To date, the Chinese traditional Baijiu industry has developed significantly, but the production of Daqu is still based on an experience-based, semi-open environment, lacking a systematic and standardized production system. This makes it difficult to fully control the quality of Daqu in industrial production and large-scale fermentation processes. The taste profile of Daqu serves as a critical metric for assessing its quality, with the microbial composition within Daqu being the primary determinant of its flavor characteristics. Therefore, research on Daqu primarily focuses on microbial communities, environmental factors, and functional strains. The enzymes within Daqu act as a bridge between the microbial community and the flavor profile of Daqu. By linking the origins of functional enzymes and their contributions to flavor synthesis, a renewed understanding of the relationship between the microbial community and the flavor of Daqu from an enzymatic perspective can be gained. In this research, a comprehensive overview of the types and production processes of Daqu were intitially provided. Subsequently, the biochemical pathways involved in the synthesis of the primary flavor compounds in Daqu were elucidated, emphasizing the roles and contributions of various functional enzymes in flavor development. Finally, contemporary techniques for enhancing Daqu flavor through microbial inoculation were examined, discussing the benefits, limitations, and current challenges associated with these methods.
There are still many problems and challenges in the development of Daqu. For example, current research on enzymes in Daqu mainly focuses on enzymes from microbial sources, but there is relatively little research on enzymes from environmental sources. A thorough understanding of enzymes from environmental sources is an important basis for achieving standardized production. In addition, the development of strengthened Daqu also faces many challenges. The production of Daqu is multidimensional, and many current studies one-sidedly pursue liquefaction power, esterification power, or fermentation power. However, in fact, the better a certain characteristic is, the higher the quality of Baijiu. Therefore, how to simultaneously suppress the growth of undesirable microorganisms, coordinate the saccharification, fermentation, and flavoring capabilities of strengthened Daqu, and reduce the generation of off-flavors is the future direction and goal for strengthening Daqu. At the same time, with the deepening understanding of functional enzymes in Daqu, using enzyme preparations to achieve targeted strengthening of Daqu is also an important direction for improving the quality of Daqu in the future.
Finally, Daqu is considered a source of functional species, valuable genes, and functional enzymes. In the future, the integration of multi-omics approaches with cultured microbiota models can facilitate the elucidation of the origin, assembly processes, and functional roles of the microbial communities in Daqu. This will provide a scientific foundation for reconstructing synthetic microbiota, thereby enhancing the quality and stability of Daqu. Through multi-omics strategies, it is possible to selectively screen and engineer key functional microorganisms and their associated enzyme genes, endowing them with more efficient substrate utilization, enhanced nutrient accumulation, the ability to degrade flavor-deteriorating factors, and improved environmental adaptability. Furthermore, the continuous refinement of multi-omics datasets not only provides the basis for rapid and precise absolute quantification of active microorganisms but also lays the groundwork for the future implementation of standardized and efficient intelligent manufacturing processes. This enhanced understanding will facilitate targeted improvements in the flavor profile, quality, and consistency of Daqu.
This work was supported by the National Natural Science Foundation of China (32272275).
-
The authors confirm contribution to the paper as follows: writing - original draft: Zhong Z; writing - review & editing: Liu T; resources: Liu J; visualization: Zhong Z; validation: Chen X, Xue Y, Han B, Liu J; data curation: He K, Zhong M; form analysis: Wang D; project administration: Liu J; supervision & funding acquisition: Liu J, Han B. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhong Z, Liu T, He K, Zhong M, Chen X, et al. 2024. Microbial enzymes: the bridge between Daqu flavor and microbial communities. Food Innovation and Advances 3(4): 426−437 doi: 10.48130/fia-0024-0041
Microbial enzymes: the bridge between Daqu flavor and microbial communities
- Received: 20 August 2024
- Revised: 25 October 2024
- Accepted: 28 November 2024
- Published online: 23 December 2024
Abstract: Baijiu Daqu, a traditional component in the Baijiu brewing process, serves as both a 'saccharifying fermenting agent' and an 'aroma-producing catalyst', embodying a rich historical legacy. Daqu offers a diverse microorganism environment that is crucial for the fermentation of Baijiu. The distinctive flavor profile, a key attribute of Baijiu, is intricately linked to the microflora present in Daqu. To date, research on Daqu has primarily concentrated on the diversity of microbial communities, microbial interactions, flavor characteristics, and biochemical properties. The functional enzyme system in Daqu serves as a crucial link connecting the flavor of Baijiu with the microbial community of Daqu. However, reviews that particularly focus on the role of enzymes in determining the quality of Daqu have not yet been reported. Thus, here the types and production processes of Daqu are initially summarized. Then, the pathways involved in the production of the major flavor substances in Daqu are elucidated, as well as the role and contribution of different functional enzymes in the formation of Daqu flavor. Finally, the current technologies for improving Daqu flavor through microbial inoculation aree discussed, including the advantages, shortcomings, and bottlenecks of microbial inoculation. The findings gained in this study provide valuable information for the efficient production of high-quality Daqu for the brewing of Baijiu.
-
Key words:
- Daqu /
- Enzyme system /
- Flavor /
- Microbial inoculation