-
According to the OIV database, global wine production is stable at around 26 billion liters, while wine consumption is around 23−25 billion liters. But 2%−5% of the world's wine was 'corked', which may cause more than one billion dollars in loss per year[1]. In the past, this problem was considered to be with corks, so it is known as 'cork taint'. We know that there are multiple causes of cork taint, and many more haloanisoles that contribute to cork taint, including 2,4,6-trichloroanisole (2,4,6-TCA), 2,3,4,6-tetrachloroanisole (2,3,4,6-TeCA), pentachloroanisole (PCA), and 2,4,6-tribromoanisiole (2,4,6-TBA)[2]. But 2,4,6-TCA is the biggest contributor to cork taint. 2,4,6-TCA is a common problem in the wine industry, producing a damaging odor commonly described by the senses as 'wet newspaper', 'damp basement', 'earthy', 'musty', and 'moldy'.
This paper will review the occurrence and formation mechanisms of 2,4,6-TCA, the detection methods of 2,4,6-TCA, and the control/remediation strategies of 2,4,6-TCA, which hope to provide a reference for the research of 2,4,6-TCA in wine.
-
Cork taint is an unavoidable problem in the wine industry. Heavy cork taint can give off a very destructive odor in wine. In a lesser level, however, it can simply blunt aromas and flavors, making a wine seem muted and uninteresting. Some studies have shown cork taint is a contaminant in wine caused by musty aroma compounds, such as multihalo-anisoles (like 2,4,6-TCA, 2,3,4,6-tetrachloroanisole (TeCA), 2,4,6-tribromoanisole (TBA), pentachloroanisole (PCA), etc), and geosmine (GSM), 2-methylisoborneol (2-MIB)[1,3]. And the most common culprit is 2,4,6-TCA. GSM and 2-MIB are more common in drinking water taste and odor problems, which are always complained about by customers. Studies show that GSM and 2-MIB are mainly produced by heterotrophic bacteria, cyanobacteria, fungi, and bryophytes[4,5]. And they usually cause earthy-musty-moldy odors. Earthy or musty sensory defects found in wine made from rotten grapes are often associated with GSM. Some studies revealed that this may be due to the presence of Penicillium expansum and other species[5].
However, among musty aroma compounds, 2,4,6-TCA is the key substance. The reason why 2,4,6-TCA is an extremely destructive odor is that, according to relevant studies, the threshold of perception of 2,4,6-TCA in water and wine is 0.03−2 ng/L and 4 ng/L respectively[6]. Secondly, 2,4,6-TCA has a certain masking effect on the perception of other aroma substances, which may be because 2,4,6-TCA enters the lipid bilayer and destroys the membrane order in the lipid microenvironment[7]. Furthermore, the activity of cyclic nucleotide-gated (CNG) channels to cilia is inhibited, which inhibits the perception of other aromas[8].
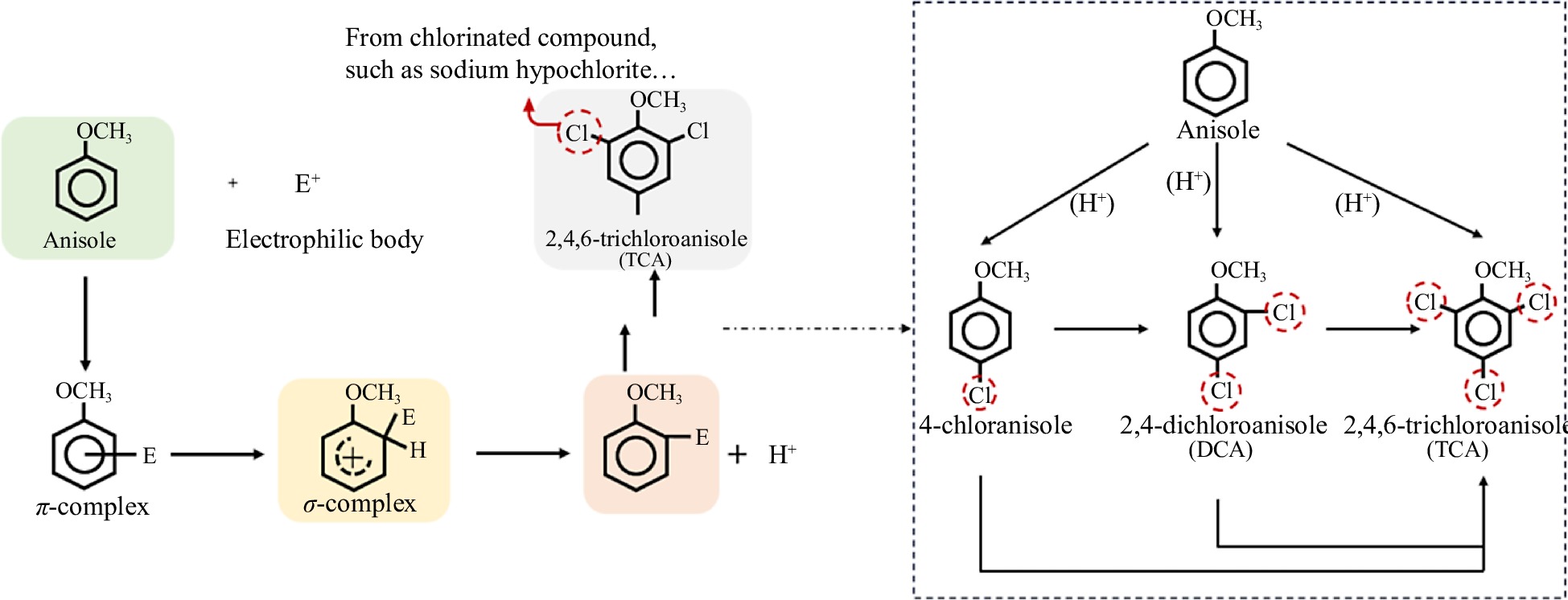
Pathway one: chlorination of anisole
-
According to the available studies, there are two main pathways for the formation of 2,4,6-TCA[9,10]. One way is the chlorination of anisole, a natural organic compound. The other is the generation of 2,4,6-TCA from the precursor substance 2,4,6-trichlorophenol (2,4,6-TCP) by microbial O-methylation. This is a typical electrophilic substitution reaction about the generation of 2,4,6-TCA from the substitution reaction of chlorine with anisole. The reaction has three main steps (Fig. 1): (1) the electrophilic body (E) attacks the benzene ring to form the π-complex and retains the benzene ring structure; (2) the electrophilic body in the π-complex attaches to a carbon atom on the benzene ring and becomes the σ-complex; and (3) a hydrogen atom bound to the benzene ring detaches and produces H+[11]. Thus, when chlorinated reagents were used, under the right conditions chlorine atoms will replace the hydrogen atoms in the benzene ring of anisole to form 2,4,6-TCA. Zhang et al. has shown that the pH determines whether the reaction occurs or not. Only under acidic and weakly acidic conditions, the substitution reaction of anisole with chlorine took place[12]. The pH of wine is usually at 3.0−4.0, and the grapes themselves are also acidic, so possessing the prerequisites for the anisole-chlorination reaction.
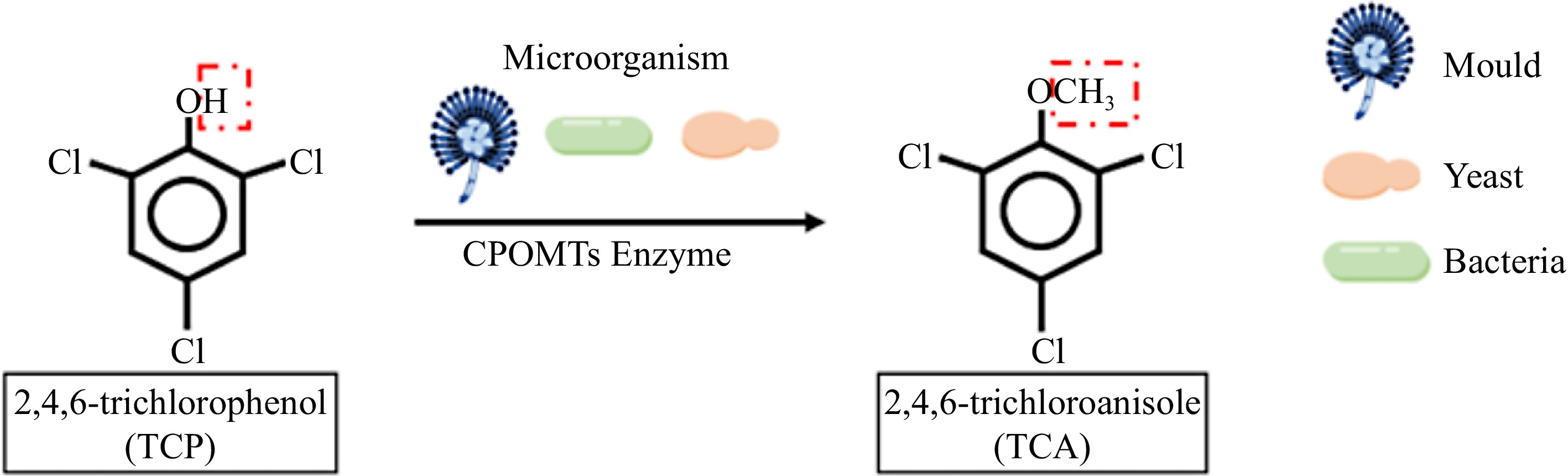
Pathway two: O-methylation of microorganisms
-
The microbial pathway for 2,4,6-TCA formation is the microbial transfer of the donor methyl group to the hydroxyl group of 2,4,6-TCP using chlorophenol O-methyltransferases (CPOMTs), which is similar to the bimolecular nucleophilic substitution reaction (SN2), in which a nucleophilic reagent attacks the substrate, provides an electron pair to the new bond, and replaces the leaving group[9,13] (Fig. 2). 2,4,6-TCP, a precursor substance in the microbial pathway formed by 2,4,6-TCA, is recognized as one of the major environmental pollutants by the US Environmental Protection Agency (USEPA). Because 2,4,6-TCP is commonly used as pesticides, herbicides, fungicides, insecticides, and disinfectants, but it is chemically stable so that it is hard to degrade, so we can often detect it in surface water, soil, and atmosphere[14,15]. The International Agency for Research on Cancer (IARC) has classified 2,4,6-TCP as a B2 carcinogen, because studies have shown that 2,4,6-TCP has significant pathological effects and potential carcinogenicity[16,17]. It has been reported that 2,4,6-TCP can affect the human nervous system and respiratory system causing diseases, such as cough and chronic bronchitis[18]. Therefore, the conversion of 2,4,6-TCP to 2,4,6-TCA by microbial action is a common biological mechanism of toxicity reduction.
Regarding CPOMTs in the microbial pathway, in terms of their methyl donors, they can be classified as S-adenosyl methionine (SAM)-dependent and non-SAM-dependent. SAM-dependent means that it only can use S-adenosyl methionine as a methyl donor, while non-SAM-dependent means that it can use a wide range of methyl donors, such as methanol, methylamine and methionine. Grapes and wine are rich in chemicals so that they can provide a rich source of methyl for the synthesis of 2,4,6-TCA.
As for the microorganisms in the microbial synthesis pathway of 2,4,6-TCA, they were mainly isolated from cork and water, because the problem of 2,4,6-TCA is mainly focused on wine cork and water. The microorganisms involved in the microbial pathway of 2,4,6-TCA formation, include bacteria, fungi, cyanobacteria and algae[10,13,19,20]. Currently the cork is still considered to be the main reason causing cork taint. Thus, studies focus on analyzing the information of fungal flora in cork and found that it is mainly composed of Penicillium spp., Aspergillus spp., Chrysonilia sitophila, Mucor racemosus, Paecilomyces spp., Trichoderma spp., Cladosporium spp., Fusarium spp., Acremonium spp., Monilia spp., Rhizoctonia spp., Mortierella spp., and Verticillium spp.[19,21,22]. However some studies revealed that Penicillium spp. (such as Penicillium chrysogenum, Penicillium glabrum), Aspergillus spp. (such as Aspergillus niger, Aspergillus oryzae), Chrysonilia sitophila, Fusarium spp., Mucor racemosus, Paecilomyces spp., and Trichoderma spp. are the main fungi, which can produce 2,4,6-TCA[19,21]. Among them, the transformation efficiency of Fusarium spp. and Trichoderma spp. strains was higher[21]. For example, one researcher isolated a SAM-dependent CPOMT from Trichoderma longibrachiatum, which can catalyze the O-methylation of several chlorophenols, including 2,4,6-TCP[23]. And study showed that its conversion efficiency was up to 37.56%. Recent research suggested that taste and odor in drinking water are mainly caused by 2,4,6-TCA. It was found that 2,4,6-TCA in water is mainly produced due to O-methylation by microorganisms. The microbial species in water are more abundant. The fungi that were found to be able to convert 2,4,6-TCP to 2,4,6-TCA were mainly dominated by Phialophora spp., Acremonium spp., and Penicillium spp.[24,25]. In addition, some bacteria, such as Gram-positive Rhodococcus spp. and Gram-negative Acinetobacter spp., can convert 2,4,6-TCP to 2,4,6-TCA[26]. Moreover, it was also found that two common cyanobacteria and algae, such as Chlorella vulgaris and Anabaena flos-aquae, can convert 2,4,6-TCP to 2,4,6-TCA[13]. From available literature, we know that the 2,4,6-TCA production capacity was significantly different between the different strains. Table 1 summarizes the current strains isolated from cork and water with the ability to convert 2,4,6-TCP to 2,4,6-TCA.
Table 1. Microorganisms associated with 2,4,6-TCA production.
Genus Species Isolated from The rate/ability of converting
TCP to TCADetection methods Ref. Fungi Acremonium Strictum Settled water 3.3%−14.24% SPME-GC-MS [27] Fungi Aspergillus − Raw one-piece cork stoppers In MEA medium, 44.5%−54.9%
On the cork, 19.9%−21.5%GC-ECD [20] Fungi Aspergillus Niger Cork stoppers On solid cork medium, 0.16%
On liquid medium, 0.65%.HS-SPME-GC-MS [19] Fungi Aspergillus Oryzae Tap water; Stoppers On solid cork medium, 0.21%;
On liquid medium, 1.17%SPME-GC-MS;
HS-SPME-GC-MS[19,27,28] Fungi Aspergillus Versicolor Settled water 40.5% SPME-GC-MS [27] Fungi Bjerkandera Adusta Finished water 2.0 × 10−5%−0.18% SPME-GC-MS [27] Fungi Botrytis Cinerea Grapes In MEA medium, 34.1%;
On wood plugs, 28.4%GC-ECD [20] Fungi Chrysonilia Sitophila Raw one-piece cork stoppers In MEA medium, 64.6%;
On wood plugs, 4.3%GC-ECD [20] Fungi Cladosporium Cladosporioides Finished water 7.0 × 10−2% SPME-GC-MS [27] Fungi Cladosporium Oxysporum Cork stoppers 14.31% HPLC [21] Fungi Cyclotella Hebeiana Lake Initially 0.2 mg/L 2,4,6-TCP; eventually 4.08 ng/L 2,4,6-TCA can be produced SPME-GC-MS [13] Fungi Fusarium Asiaticum Finished water 0.28% SPME-GC-MS [27] Fungi Fusicolla Matuoi Finished water 2.6% SPME-GC-MS [27] Fungi Fusarium Oxysporum Cork stoppers 28.65% HPLC [21] Fungi Laccaria Amethystina Raw water;
Settled water;
Post filtration water; Finished water2.9 × 10−2% SPME-GC-MS [27] Fungi Mortierella Alpina Cork stoppers 0.11% HPLC [21] Fungi Mucor Plumbeus Cork stoppers 0.03% HPLC [21] Fungi Mucor Racemosus Cork stoppers On solid cork medium, 5.21%;
On liquid medium, 5.21%HS- SPME-GC-MS [19] Fungi Paecilomyces Variotii Fibreboard cartons 2%−65% HPLC; GC-MS [29] Fungi Paecilomyces Viridis Cork stoppers 7.88% HPLC [21] Fungi Paecilomyces − Cork stoppers On solid cork medium, 3.65%;
On liquid medium, 4.45%HS- SPME-GC-MS [19] Fungi Penicillium − Raw one-piece cork stoppers In MEA medium, 23.2-37%;
On cork, 1.3%−53.1%GC-ECD [20] Fungi Penicillium Chrysogenum Cork stoppers 3.29%−7.87% HS- SPME-GC-MS [19] Fungi Penicillium Citreonigrum Cork stoppers 13.28% HPLC [21] Fungi Penicillium Decumbens Cork stoppers 0.11% HPLC [21] Fungi Penicillium Glabrum Cork stoppers 2.18%−20.43% HS- SPME-GC-MS [19] Fungi Penicillium Purpurogenum Cork stoppers 11.02% HPLC [21] Fungi Phialemoniopsis Ocularis Post filtration water 0.13% SPME-GC-MS [27] Fungi Pseudomonas − Lake – SPME-GC-MS [13] Fungi Rhizopus Oryzae Broiler house litter < 1% Gas-Liquid Chromatography [30] Fungi Scopulariopsis Brevicaulis Broiler house litter 60% Gas-Liquid Chromatography [30] Fungi Sistotrema Brinkmannii Post filtration water; Finished water 2.3% SPME-GC-MS [27] Fungi Talaromyces Pinophilus Finished water 2.7% SPME-GC-MS [27] Fungi Trichoderma − Raw one-piece cork stoppers; Lake In MEA medium, 64.4%;
On cork, 13%GC-ECD; SPME-GC-MS [13,20] Fungi Trichoderma Longibrachiatum Cork 37.56% – [31] Fungi Trichoderma Viride Cork stoppers 3.37%−4.86% HS- SPME-GC-MS [19] Fungi Verticillium Psalliotae Cork stoppers 6.9% HPLC [21] Bacteria Acinetobacter − Water 2.4 × 10−10 ug*h (cell/mL) GC-MS [32] Bacteria Bacillus Australimaris Water OMPPC (1.31 × 10−9 ng/CFU) SPME-GC-ECD [33] Bacteria Brachybacterium Brachybacterium Cork – HS-SPME-GC-MS [32] Bacteria Brachybacterium Paraconglomeratum Cork – HS-SPME-GC-MS [32] Bacteria Bradyrhizobium Frederickii Water produce 2,4,6-TCA
(1.7 × 10−9 ng/CFU)SPME-GC-ECD [33] Bacteria Brevundimonas − Water – SPME-GC-ECD [33] Bacteria Caulobacter − Water – SPME-GC-ECD [33] Bacteria Chromobacterium − Water – SPME-GC-ECD [33] Bacteria Erythrobacter − Water – SPME-GC-ECD [33] Bacteria Escherichia Coli Lake Initial 0.2 mg/L 2,4,6-TCP;
generated 4.6 ng/L 2,4,6-TCASPME-GC-MS [13] Bacteria Flavobacterium − Cork – HS-SPME-GC-MS [32] Bacteria Microbacterium Oxydans Cork – HS-SPME-GC-MS [32] Bacteria Paenibacillus − Water – SPME-GC-ECD [33] Bacteria Pelomonas − Water – SPME-GC-ECD [33] Bacteria Ralstonia Mannitolilytica Water – SPME-GC-ECD [33] Bacteria Rhodoccoccus Acinetobacter Lake – SPME-GC-MS [13] Bacteria Rhodococcus − Water 5.5 × 10−8 ug*h (cell/mL) GC-MS [34] Bacteria Xanthobacter − Water – SPME-GC-ECD [33] Cyanobacteria Chlorella Vulgaris Lake Initial 0.2 mg/L 2,4,6-TCP;
generated 30.5 ng/L 2,4,6-TCASPME-GC-MS [13] Algae Anabaena Flos-aquae Lake Initially 0.2 mg/L 2,4,6-TCP;
generated 10.2 ng/L 2,4,6-TCASPME-GC-MS [13] -
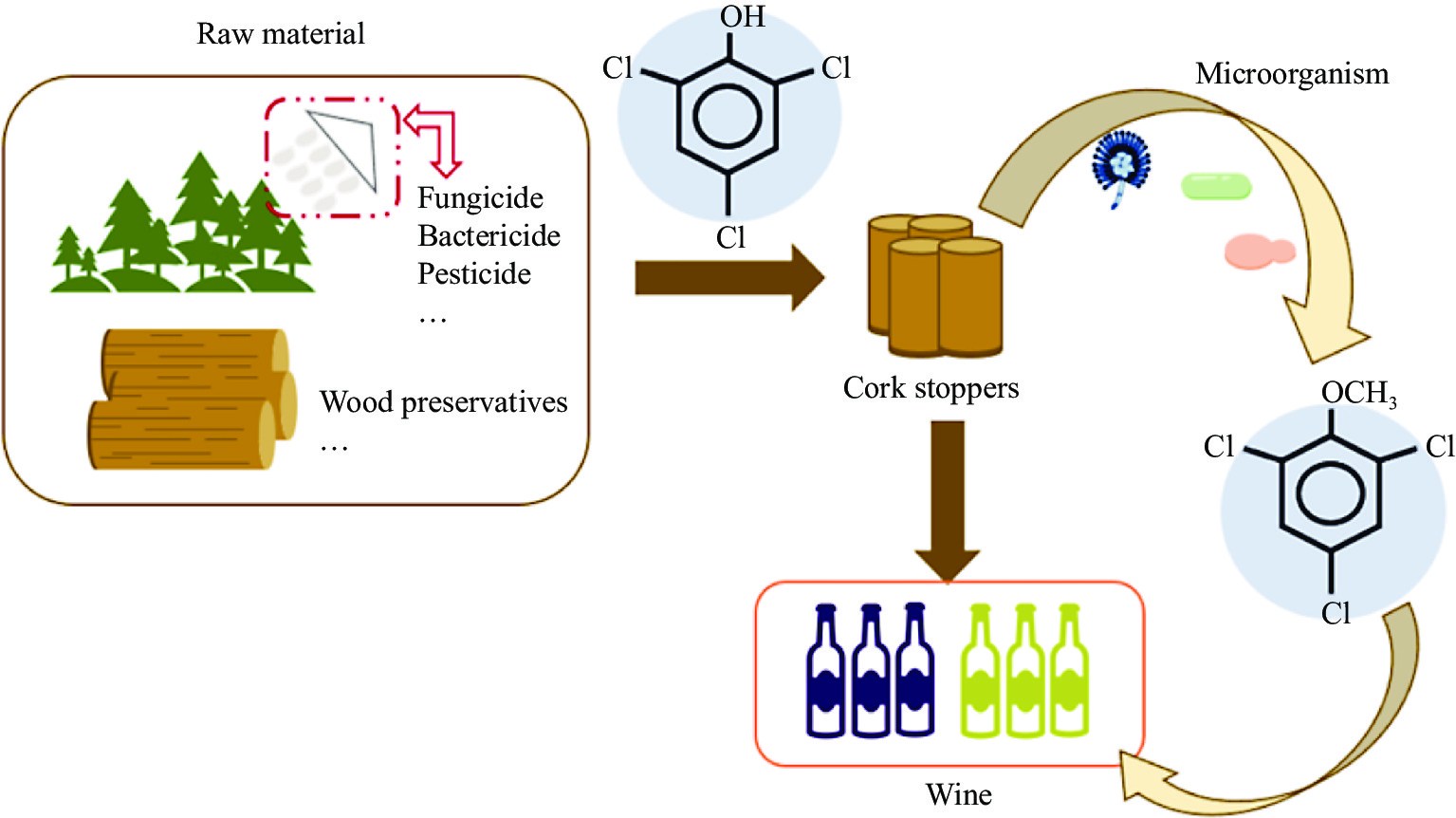
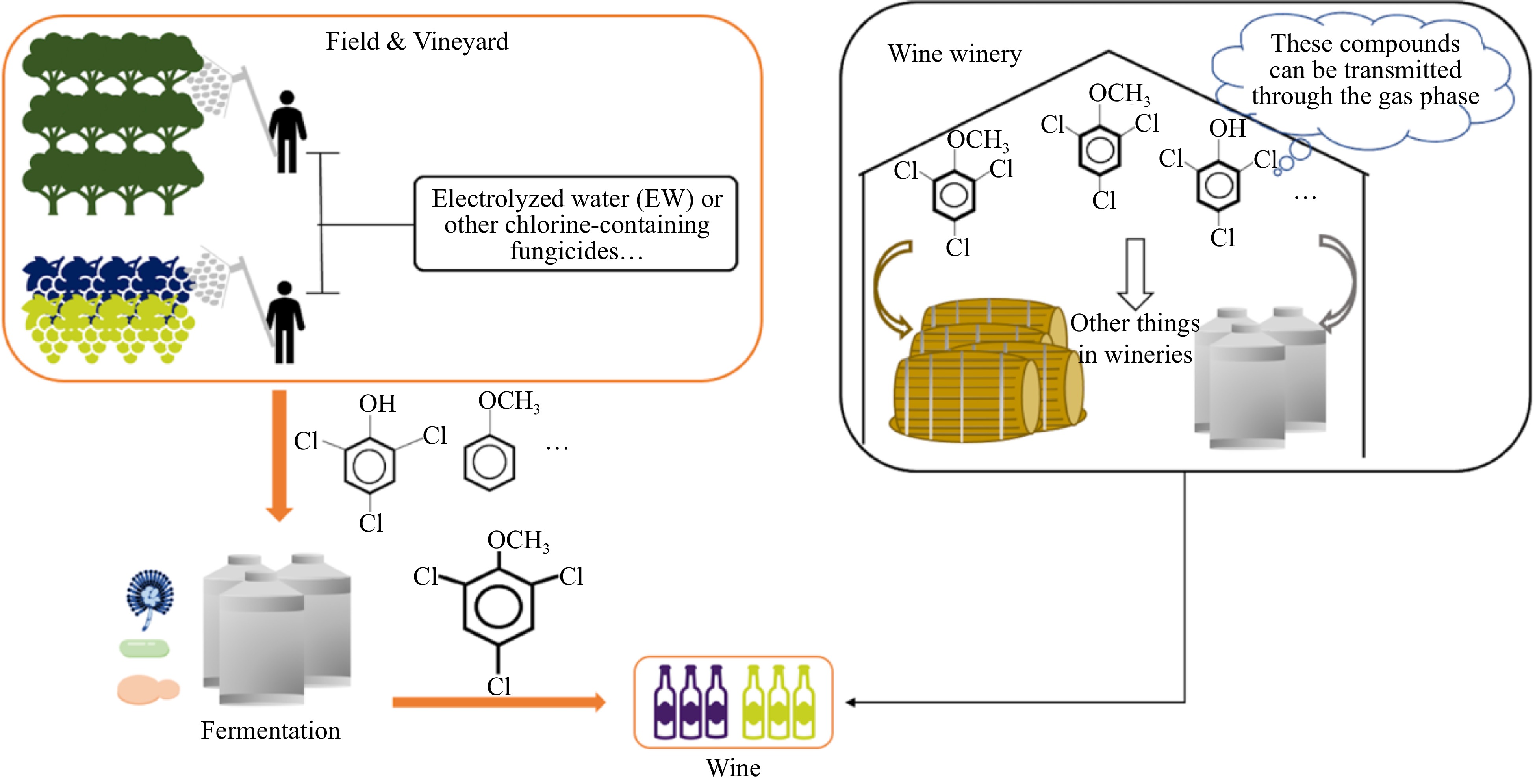
In the early 1980s, the source of cork taint was identified as microbial contamination of cork, and the residual chlorophenol compounds from pesticides during oak growth and wood preservatives, which in turn produce 2,4,6-TCA. When the cork comes into contact with the wine, 2,4,6-TCA can be transferred from the cork to the wine (Fig. 3). As we all know, cork is mainly made from the bark of cork oak, due to its good elasticity, it can play a good role in sealing the mouth of the bottle. Moreover, there are tiny spaces between the cork cells, which cannot completely isolate the air, so facilitating the slow development and maturation of the wine in the bottle again. Besides, when the cork is in direct contact with the wine, some components in the cork can be transferred to the wine, such as phenolic compounds, tannins, and ketones[35,36]. According to the statistical report of the International Organization of Vine and Wine (OIV), 70% of the world's total wine production of bottled wine is sealed with cork[37]. Therefore, cork is currently considered the main culprit of 2,4,6-TCA taint in wine. Monteiro et al. showed that cross-contamination of cork can occur through both the liquid and gas phases, i.e., a cork contaminated with 2,4,6-TCA is partially contaminated with a clean cork immersed in either pure water or an alcohol solution. And the contaminated cork with clean storage for some time, the clean cork will likewise be partially contaminated[38]. Therefore, methods that can quickly and non-destructively detect whether a cork is contaminated with 2,4,6-TCA are much needed. Moreover, 2,4,6-TCA contamination can be transmitted through the gas phase, suggesting that 2,4,6-TCA can contaminate wine through the air from other woods, such as oak barrels, cellar beams, and wood chips.
Water
-
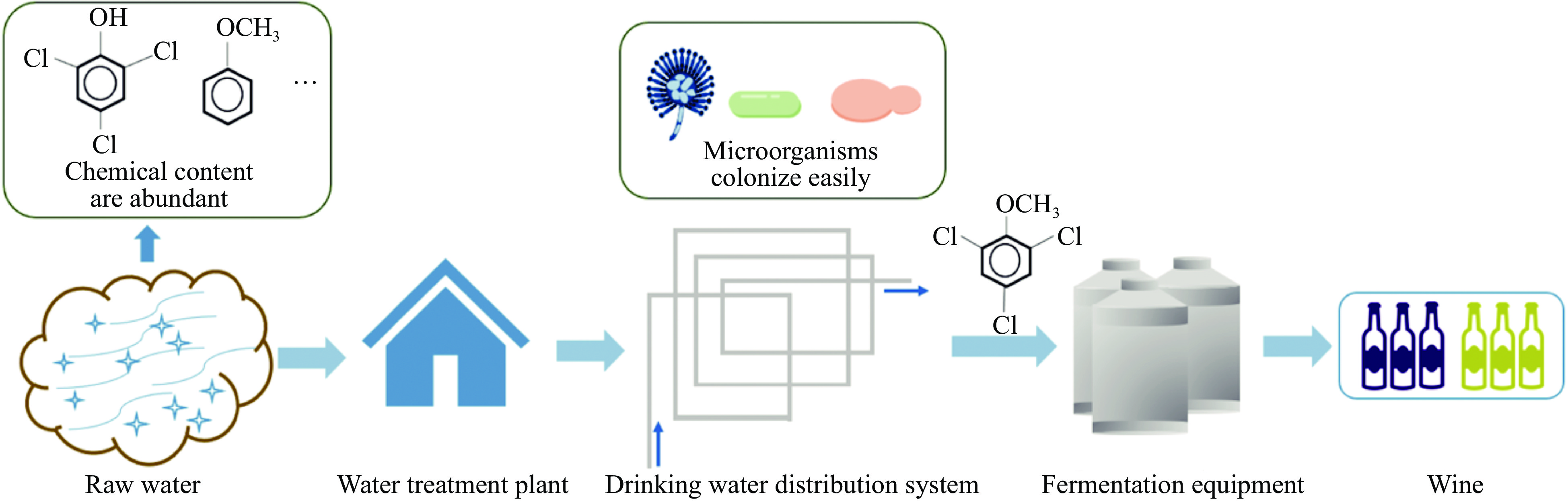
The odor problem of drinking water is also often complained about by consumers, studies have found that the substances causing these odors are mainly GSM, 2-MIB, and 2,4,6-TCA. The formation of 2,4,6-TCA in water is mainly through microbial O-methylation, because the chlorination of anisole occurs only under acidic conditions, while the pH of drinking water usually does not present acidity. Therefore, even if 2,4,6-TCA was effectively removed from the source water, 2,4,6-TCA would still be generated in drinking water[9,10,12]. In addition to SAM, other methyl donors, including methanol and methylamines are present in water in the form of natural organic matter[9]. In the drinking water distribution and delivery system, microorganisms tend to grow in the pipes, so it is easier to generate 2,4,6-TCA. Water is usually used in the winemaking process, so it may also be a source of 2,4,6-TCA in wine (Fig. 4).
Others
-
Recently, electrolyzed water (EW) has attracted a lot of attention as a new high-performance technology for potential applications in the food industry. EW has a certain disinfection effect on microorganisms and is extremely promising as a bactericidal agent with a wide range of disinfection effects and eco-friendliness[39,40]. Some studies have shown the bactericidal potential of EW against wine spoilage yeasts, e.g., Brettanomyces spp.[41]. However, some studies have found that both pre-harvest and post-harvest applications of EW increase the concentration of 2,4,6-TCA in wine[42,43]. Previous research explained that EW application leads to chlorine residues on the grape surface, which in turn produce 2,4,6-TCA in response to microbial action. This suggests that the use of chlorinated fungicides during grape growing is also a source of 2,4,6-TCA in wine. Therefore, we need to use fungicides properly.
Monteiro et al. suggested that 2,4,6-TCA contamination can be transmitted through the gas phase[38]. Once 2,4,6-TCA forms in wooden materials inside the cellar or winery, it can migrate into the air and contaminate winery equipment and oenological materials. Finally, it can lead to cork taint (Fig. 5). Some researchers simulated air contamination (initial d5-TCA concentration was 50 ng/L of air) and stored the sealed wine for 6 to 24 months[44]. They found that these wines were at risk of contamination. Another study also reported that sparkling wine sealed with crown caps was contaminated by airborne tetrachloroanisole after 14 months of storage.
-
The detection of 2,4,6-TCA is challenging due to its concentration in wine, which is usually at the ng/L level, and the complexity of matrices such as wine and cork. To address this challenge, researchers have developed chromatography-based or bioanalytical techniques for the detection of 2,4,6-TCA in various matrices such as cork, wine, and water. Usually, gas chromatography-mass spectrometry (GC-MS) and gas chromatography-electronic capture detector (GC-ECD) are used to detect 2,4,6-TCA concentration.
GC-MS is a common detection tool in the field of wine research and can be used for qualitative analysis as well as quantitative analysis. GC-MS can achieve high separation efficiency for the identification and quantification of aroma substances, such as haloanisoles, esters, and terpenes[45]. Tarasov et al. determined 2,4,6-TCA content in wine by GC-MS combined with solid phase microextraction (SPME), and its limits of detection (LODs) can achieve 0.4 ng/L[44]. Wines contain a variety of substances, so the detected sample matrix is complex. Thus, the background noise of the detection using GC-MS is large. Some researchers use GC-MS/MS, which is highly selective and sensitive to compounds compared to GC-MS, and it can better detect multiple substances with similar structures simultaneously. Zhang et al. used GC-MS/MS coupled with headspace solid phase microextraction (HS-SPME) to determine nine multihalo-anisoles (such as 2,3,4,5-TeCA, 2,3,4,6- TeCA, PeCA, TBA, 2,4,6-TCA) and multihalo-phenols (such as PeCP, TBP, TCP, TeCP) in wine, and its LODs can achieve within 3.0 ng/L[46]. Ruiz-Delgado et al. also determined cork contaminants in wine by GC-MS/MS combined with HS-SPME, and the LODs for 2,4,6-TCA, 2,3,4,6-TeCA, 2,4,6-TBA, and PCA in wine were less than 0.3 ng/L[47].
ECD is an ion detector which is highly sensitive to compounds containing electronegative elements. Chloroanisole and its precursor chlorophenols contain multiple chlorine atoms, which are electronegative, so the ECD was chosen to detect them with good selectivity and high sensitivity. Özhan et al. assayed the levels of 2,4-dichloroanisole (DCA), 2,4,6-TCA, 2,3,4,6-TeCA, PCA, 2,4,6-TCP, 2,3,4,6-TeCP, PCP in red wine from different wineries in Turkey using HS-SPME and GC-ECD detection, and the LODs were less than 1.0 ng/L[48]. In addition, compared to GC-MS/GC-MS/MS, GC-ECD has a low purchase price and maintenance cost. However, ECD is mainly suitable for halogenated cork-taint compounds.
Meanwhile, a large number of studies related to the separation and identification of odor-active compounds in food by gas chromatography-olfactometry (GC-O) have been carried out. Some studies screened and identified the odor-active compounds in ice wines by GC-O combined with comprehensive two-dimensional GC and time-of-flight mass spectrometry (GC
$ \times $ Ion Mobility Spectrometry (IMS) is an analytical technique for characterizing molecules by gas phase mobility, which has the advantage of rapid detection, high sensitivity, and the ability to avoid interference from other compounds present in the matrix and is often used for the detection of various explosives, drugs and narcotics[49,50]. In addition, ion mobility spectrometers are relatively inexpensive and can provide spectra in the millisecond range. These advantages make IMS suitable for the detection of volatile or semi-volatile compounds in different matrices. But its selectivity is limited, extraction and preconcentration of 2,4,6-TCA from wine samples is necessary. Because there are interfering substances in wine samples, mainly ethanol, which can overlap with the signal of 2,4,6-TCA. Thus, Márquez-Sillero et al. firstly used solid-phase extraction to remove ethanol. They then combined the use of ionic-based single drop microextraction (ILSDME) and IMS for the determination of 2,4,6-TCA in water and wine samples. This method LOD is 0.2 ng/L[51]. The next year, they developed a new method based on IMS that the interference of ethanol was negligible. They analyzed 2,4,6-TCA in wine and cork samples by headspace-multicapillary column-ion mobility spectrometry (HS-MCC-IMS), and the detection limit of wine is 0.012 ng/L, the detection limit of cork is 0.28 ng/L[52]. It greatly improves the sensitivity of the detection method.
As mentioned above, 2,4,6-TCA can also cross-contaminate through the gas phase, which means 2,4,6-TCA may also be present in the ambient air of the winery. Thus, it is important to detect 2,4,6-TCA in the environment early in the winemaking process to prevent cork taint of wine. Therefore, a method based on thermal desorption coupled to GC-MS (TD-GCMS) was proposed for the determination of low concentrations of the target compounds in the air, using a porous polymer resin based on 2,6-diphenylene oxide as an adsorbent instead of bentonite, which was used in the past to capture target compounds in the air[53].
Electronic nose
-
However, GC-MS, GC-MS/MS, or GC-ECD needs a previous step of sample preparation, which usually is destructive and time-consuming, and sometimes requires using organic solvents. Among the available techniques, the electronic nose stands out. The electronic nose (Enose), also known as an odor scanner is a novel instrument developed in the 1990s for rapid food testing. It is an instrument consisting of a set of chemical gas sensors with partial specificity and an appropriate pattern recognition system, capable of recognizing simple or complex odors[54]. Santos et al. investigated the feasibility of a small wireless portable nose (WiNOSE 6), composed of non-specific cross-sensitivity sensors, capable of measuring up to eight microsensors to detect typical and atypical odor compounds in natural cork[55]. Corks were introduced in a 50 mL vial with two holes at the top, one for atmospheric air and the other connected to a nasal cannula. Each measurement cycle consisted of a 9-min desorption phase and a 1-min adsorption phase. And results showed close to 100% identification of defects such as MDMP, TCA, and 1-octene-3-one. Melendez et al. also present a prototype of a novel Enose that uses an array of digital and analog metal oxide gas sensors with a total of 31 signals capable of detecting 2,4,6-TCA and classifying cork samples with low 2,4,6-TCA concentrations (
$ \le $ Cyclic voltammetry
-
Since GC methods often require pretreatment of the sample to be measured, and the instruments are also more expensive and sophisticated, often requiring specialized personnel to operate. Peres et al. quantified 2,4,6-TCA in cork plates using cyclic voltammetry (CV), a commonly used electrochemical research method to study the nature, mechanism, and kinetic parameters of electrode reactions, and also for quantitative determination of reactant concentrations[57]. It works by applying a pulsed voltage in the form of an isosceles triangle to the working electrode and controlling the electrode potential at different rates with one or more repeated scans of the triangular waveform over time to obtain a current-potential polarization curve. Sanvicens et al. used a portable Potentiostat-Galvanostat device (PG580, Uniscan) together with a silver working electrode (M295Ag, Radiometer), a platinum counter electrode (M241Pt, Radiometer) and an Ag/AgCl double-junction reference electrode (M90-02, Orion) for measuring the current of sample collected from the cork plank boiling process[57]. In addition, CV devices are portable, fast, and low-cost, and do not require specialized technicians, so making them promising for field industrial applications.
Enzyme-linked immunosorbent assays and electrochemical immunosensing
-
To improve the sample quantity or test cycle, speed and reduce cost, some emerging rapid assays have been proposed, such as enzyme-linked immunosorbent assays (ELISAs)[58] and immunoamperometric assays[59]. ELISAs are an immunoassay method that combines the high specificity of antigen-antibody reaction with the high efficiency of enzyme catalysis, mainly based on the ability of antigens or antibodies to adsorb onto the surface of the solid-phase carrier and maintain its immunological activity, and then use the specific binding of antigens and antibodies for the qualitative and quantitative detection of immunological reactions. Lausterer et al. prepared antibodies specific for TCA by fused cells and used the Rami kit for ELISA detection of signal amplification up to 10 ng/L[60]. They synthesized haptens B (5-(2,4,6-trichlorophenoxy)pentanoic acid) and C (3-(3,5-dichloro-4-methoxyphenyl)propanoic acid) by chemical methods and conjugated with bovine serum albumin and keyhole limpet hemocyanine by the active ester method, respectively. Then an immune response is induced by injecting these synthesized compounds into immunized animals, such as mice. Subsequently, lymphocytes were collected from immune animals and fused with myeloma cells to form hybridoma cells. Then hybridoma cells with 2,4,6-TCA selectivity were screened by immunization and fusion. Finally, two different cell lines (Rami and Hbab) were selected from the selected hybridoma cells, cloned, and stored at low temperatures. However, ELISAs methods usually require preparative steps such as extraction and concentration, which increase the analysis time. Therefore, electrochemical immunosensing technique was developed for 2,4,6-TCA detection. This technique can avoid sample interferences. Apostolou et al. based the team on a previous bioelectric recognition assay (BERA) biosensor system, which is based on the determination of the electrical response of cultured membrane-engineered fibroblasts suspended in an alginate gel matrix[61]. High-throughput screening of TCA in cork was achieved by osmotically inserting a specific TCA antibody (pAb78) into the cork. This new method can detect very low concentrations of 2,4,6-TCA (down to 0.2 ng/L) in just 5 min. This new biosensor offers several practical advantages, including a significant reduction in total assay time and the ability to perform high-throughput screening directly in the field and production facilities without the need for any support infrastructure. However, this method does not provide reliable quantitative results and only detects a small fraction of the concentration in the sample due to the extremely low solubility of 2,4,6-TCA in water[61].
Others
-
Recently, Romano et al. tested a new method for the determination of 2,4,6-TCA in cork based on chemical ionization time-of-flight mass spectrometry (CI-TOF) using a 'Vocus' ion source and an ion-molecule reactor (IMR), which allowed a rapid and highly sensitive detection of 2,4,6-TCA in coffee beans within 3 s[6]. And they suggested that the method is also feasible for other food products. Cappellin et al. simulated a real industrial scenario and determined the 2,4,6-TCA content of 10,100 natural cork batches in three different batches in just 8 hours and 25 min, which is equal to 3 s per cork[62]. This method far exceeds existing analytical methods in terms of speed and has approximately the same detection limits as other assays. Therefore, this new non-destructive, rapid, and sensitive detection technique has the potential to be a breakthrough for the cork and wine industry. Based on the article, it can be hypothesized that the technique can detect other pollutants. However it is not possible to determine the applicability of the technique for the simultaneous detection of multiple contaminants.
Damiano et al. developed a method based on Ni(0) complexes to detect 2,4,6-TCA in cork indirectly by UV-Vis spectroscopy, since aryl chlorides can effectively participate in the oxidative addition reaction with phosphorylated Ni(0)(BINAP) (
$ \eta $ Pre-treatment methods
-
The key to the analysis of 2,4,6-TCA in wine is sample preparation with pre-enrichment or extraction in advance. The common pretreatment methods reported in domestic and international research includes head space solid microextraction (HS-SPME)[64], stir bar sorptive extraction (SBSE)[65], dispersive liquid-liquid microextraction (DLLME)[66,67], supercritical fluid extraction (SFE)[68], accelerated solvent extraction (ASE), and pressurized fluid extraction (PFE)[69]. These different extraction techniques combined with GC-MS and other detection techniques have been successfully used to identify 2,4,6-TCA in wine, cork, or water[46].
Solid microextraction (SPME)
-
SPME is the most commonly reported technique for sample extraction or pre-enrichment. Reported methods based on SPME combined with different instruments for the detection of typical odorants are summarized in Table 2. The SPME method uses a fibrous membrane coated with an extraction phase (liquid polymer or solid adsorbent) to extract different types of analytes (volatile or non-volatile substances) from various media (liquid or gas phase)[70]. SPME methods are easy to perform and can greatly reduce environmental contamination by the use of organic solvents, and can make the LOD of the method as low as ng/L. SPME methods can be carried out either by direct immersion in liquid samples (DI-SPME) or the more commonly used headspace method (HS-SPME). Jové et al. used HS-SPME and GC–MS/MS to detect 2,4,6-TCA, 2,3,4,6-TeCA, 2,4,6-TBA, and PCA in cork stoppers. Results showed that the divinylbenzene/carboxenpolydimethylsiloxane/polydimethylsiloxane (DVB/CAR/PDMS) fibers could detect haloanisoles with the LODs at 0.01–0.50 ng/L[3]. However, we need to further develop and optimize the procedure for different situations such as different solvent types to improve the extraction efficiency and accuracy of the results.
Table 2. Analysis methodology regarding 2,4,6-TCA.
Microextraction methodologies Detection methodologies LOD LOQ Analysis time per sample Ref. Instrument Sample type Analytes Fiber type Extraction condition Instrument Column type GC condition MS condition Internal standards HS-SPME Wine d5-TCA PDMS,
100 μmIncubation
temperature: 55 °C
Incubation time: 3 min
Sample extraction time: 11 min
Sample desorb time:
4 minQP-2010 Plus GC-MS (Shimadzu, Kyoto, Japan) RTX-5MS Gas flow 1.61 mL/min
Oven program:
90 °C for 0 min,
10 °C/min to 205 °C, and then 30 °C/min to 280 °CSIM d5-TBA 0.4 ng/L 1 ng/L 32 min [44] HS-SPME Wine TCA, TCP, 2,3,4,6-TeCA, TBA, 2,3,4,6-TeCP, 2,3,4,5-TeCA, PeCA, TBP, PeCP DVB/CAR/
PDMS,
50/30 µmIncubation
temperature: 60 °C
Incubation time: 5 min
Sample extraction time: 45 min
Sample desorb time:
5 minAgilent 7890A
GC-7000B triple quadrupole MSHP-5 Flow rate: 1.18 mL/min
Oven program:
50 °C for 1 min,
10 °C/min to 200 °C, and then 40 °C/min to 280 °C hold for 3 minMS/
MS-MRMTCA-d5 Haloanisoles:
3 ng/L
Halophenols:
10 ng/LHaloanisoles:
10 ng/L
Halophenols: 30−100 ng/L76 min [46] HS-SPME Wine TCA, TeCA, TBA, PCA PDMS,
100 μmIncubation
temperature: 40 °C
Incubation time: 5 min
Sample extraction time: 30 min
Sample desorb time:
15 minScionGC system (Bruker Corporation, Freemont, CA, USA) × Scion QqQ-MS/MS instrument (Bruker) VF-5ms Flow rate: 1 mL/min
Oven program:
90 °C for 5 min,
30 °C/min to 280 °C hold for 7 minSRM 4-iodoanisole TCA: 0.1 ng/L
TeCA: 0.2 ng/L
TBA: 0.3 ng/L
PCA: 0.1 ng/LTCA: 0.4 ng/L
TeCA: 0.6 ng/L
TBA: 0.9 ng/L
PCA: 0.3 ng/L68.33 min [47] HS-SPME Cider TCA, TeCA, TBA, PCA PDMS,
100 μmIncubation
temperature: 40 °C
Incubation time: 5 min
Sample extraction time: 30 min
Sample desorb time:
15 minScionGC system (Bruker Corporation, Freemont, CA, USA) × Scion QqQ-MS/MS instrument (Bruker) VF-5ms Flow rate: 1 mL/min
Oven program:
90 °C for 5 min,
30 °C/min to 280 °C hold for 7 minSRM 4-iodoanisole TCA: 0.2 ng/L
TeCA: 0.2 ng/L
TBA: 0.3 ng/L
PCA: 0.1 ng/LTCA: 0.5 ng/L
TeCA: 0.7 ng/L
TBA: 1.1 ng/L
PCA: 0.5 ng/L68.33 min [47] HS-SPME Cava TCA, TeCA, TBA, PCA PDMS,
100 μmIncubation
temperature: 40 °C
Incubation time: 5 min
Sample extraction time: 30 min
Sample desorb time:
15 minScionGC system (Bruker Corporation, Freemont, CA, USA) × Scion QqQ-MS/MS instrument (Bruker) VF-5ms Flow rate: 1 mL/min
Oven program:
90 °C for 5 min,
30 °C/min to 280 °C hold for 7 minSRM 4-iodoanisole TCA: 0.1 ng/L
TeCA: 0.2 ng/L
TBA: 0.4 ng/L
PCA: 0.2 ng/LTCA: 0.4 ng/L
TeCA: 0.6 ng/L
TBA: 1.3 ng/L
PCA: 0.7 ng/L68.33 min [47] HS-SPME Water TCA, TCP PDMS,
100 μm– GC-2010/parvum 2, Shimadzu, Kyoto, Japan 5MS/Sil Flow rate: 42 cm/s
Oven program:
40 °C for 3 min,
10 °C/min to 80 °C, and then 15 °C/min to
250 °C hold for 3 minSIM – – – > 21.3 min [74] HS-SPME Water 2-CP, 2-BP, 2,4-DCP, 2,4,6-TCP, 2,4-DBP, 2,4,6-TBP, 2,4,6-TCA, 2,4,6-TBA PDMS/DVB,
65 μmIncubation
temperature: 60 °C
Incubation time: 10 min
Sample extraction time: 30 min
Sample desorb time:
3 minAgilent 7890 GC × Agilent 5975 MS HP-5MS Flow rate: 1 mL/min
Oven program:
40 °C for 3 min,
15 °C/min to 235 °C hold for 1 minSIM 4-iodoanisole Haloanisoles: 0.23−0.29 ng/L
Halophenols: 0.24−0.91 ng/LHaloanisoles: 0.97−0.77 ng/L
Halophenols: 0.80−3.30 ng/L60 min [75] HS-SPME Garlic TCA, TBA CWR/PDMS, 120 µm Incubation
temperature: 80 °C
Sample extraction
time: 20 minShimadzu GC-2010 × Shimadzu TQ8050 Rxi-5 ms Flow rate: 35 cm/s
Oven program:
70 °C for 1 min,
10 °C/min to 300 °C hold for 3 min.MS/MS-MRM TCA-d5 – TCA:
0.02 μg/kg
TBA:
0.03 μg/kg47 min [76] SPME Water 2,4,6-TCA, 2,3,6-TCA, 2,3,4-TCA, 2,4,6-TBA PDMS/DVB/
CAB,
50/30 µmIncubation temperature: 70 °C
Incubation time: 10 min
Sample extraction time: 30 min
Sample desorb time:
10 minGC-MS HP-17MS Oven program:
45 °C for 4 min,
10 °C/min to 240 °C hold for 1 min,
30 °C/min to 280 °C
hold for 4 minSIM – 2,4,6-TCA:
0.098 ng/L
2,3,6-TCA:
0.127 ng/L
2,3,4-TCA:
0.109 ng/L
2,4,6-TBA:
0.086 ng/L– 79.8 min [77] Vacuum-assisted HSSPME Wine TCA, TeCA, PCA, TBA PDMS/DVB,
65 μmIncubation
temperature: 25 °C
Incubation time: 10 min
Sample extraction time: 30 min
Sample desorb time:
15 minShimadzu GC-17 A, GC-ECD DB-5MS Flow rate: 1 mL/min
Oven program:
90 °C for 5 min,
20 °C/min to 280 °C hold for 5 min– – TCA 0.16 ng/L
TeCA 0.18 ng/L
PCA 0.19 ng/L
TBA 0.13 ng/L– 74.5 min [78] LLE Wine TCA, TeCA, PCA, TBA, TCP, TeCP, PCP, TBP – – Agilent HP 5980 GC × ECD (Agilent Technologies, USA) CP-Sil 5CB Oven program:
40 °C for 0 min,
3 °C/min to 160 °C, and then 5 °C/min to
220 °C hold for 10 min– – – – >
38 min[79] Pressurized liquid extraction Cork MDMP, IPMP, IBMP, TCA, TCP, TeCA, TeCP, TBA, TBP, PCA – – Agilent 6890 N GC × Agilent 5973 N MS DB-5 Flow rate: 1 mL/min
Oven program:
40 °C for 10 min,
2 °C/min to 155 °C, and then 20 °C/min to
260 °C hold for 9 minSIM 2,3,6-trichloroanisole 0.10 ng/g – > 81.75 min [80] DLLME Wine 2-CA, 4-CA, 2-BA, 2,6-DCA, 2-CP, 4-BA, 4-CP, 2-BP, 2,4-DCA, 4-BP, 2,6-DCP, 2,4,6-TCA, 3M4CP, 2,4-DCP, 2,4,6-TCP, 2,4-DBA, 2,3,4,6-TeCA, 2,4-DBP, 2,4,6-TBA, 2,3,4,6-TeCP, 2,3,4,5-TeCA, PCA, 2,4,6-TBP, PCP – – Agilent 6890 N GC × Agilent 5973 MS HP-5MS Flow rate: 1 mL/min
Oven program:
40 °C for 5 min,
5 °C/min to 105 °C hold for 3.5 min,
5 °C/min to 120 °C hold for 3 min,
10 °C/min to 145 °C,
and then
5 °C/min to 185 °C,
10 °C/min to 200 °C
hold for 0.5 minSIM – 0.006–0.05 ng/mL – 40 min [81] – Cork TCA, TeCA, TBA, PCA – – Agilent 6890N GC × Agilent 5973 MS HP-5MS Flow rate: 1 mL/min
Oven program:
80 °C for 0.6 min,
25 °C/min to 180 °C hold for 0.6 min
25 °C/min to 210 °C
hold for 0.8 min,
50 °C/min to 300 °C
hold for 1.4 minSIM 5-Bromo-2-chloroanisole TCA: 1.6 ng/g
TeCA: 2.6 ng/g
TBA: 1.7 ng/g
PCA: 2.5 ng/gTCA: 5.4 ng/g
TeCA: 8.8 ng/g
TBA: 5.7 ng/g
PCA: 8.5 ng/g> 9.6 min [82] Stir bar sorptive extraction (SBSE)
-
SBSE is a method of extracting a target substance by stirring and contacting a sample solution using a stir bar with a specific fiber coating. After adsorption of the target substances in the sample onto the fiber coating, the fibers are fed into GC-MS for analytical determination. SBSE can extract a large amount of solution, and the extracted target substances can be immobilized by the adsorbent material in the stir bar for a sufficient period, which makes it convenient for on-site sampling and transportation[71]. Moreover, SBSE is very effective for trace components because the extraction phase is relatively large (about 5 μL for 10 mm) compared to that of SPME (about 65 μL for 100 μm)[72]. SBSE is also selective and can selectively adsorb target compounds, thereby reducing the effect of interfering substances. Marsol-Vall et al. used SBSE and heart-cutting two-dimensional gas chromatography to detect halophenols and haloanisoles in cork bark macerates. Results showed that the method gave LODs and LOQs ranging from 0.03 to 0.24 ng/L[72].
Dispersive liquid-liquid microextraction (DLLME)
-
Liquid-liquid extraction is one of the most classical pretreatment methods and has been widely used for the analysis of various matrix parameters. DLLME has been developed since 2006, which has the characteristics of simple operation, high enrichment factor, and low consumption of organic solvents. In DLLME, an organic solvent (extraction solvent) is dispersed into an aqueous sample with the help of a co-solvent, the dispersant. The dispersion permits the formation of a large contact surface between the sample and the extractant, thus facilitating the extraction of analytes from the organic phase. Pizarro et al. used a method based on DLLME combined with GC-MS/MS technique to analyze compounds responsible for cork-taint off-flavors in wine[73]. Results showed that the method gave LODs and LOQs ranging from 5 to 41 ng/L. Despite the many advantages of DLLME methods, such as economy, simplicity, and rapidity, there are still some disadvantages and application limitations. The key to DLLME methods lies in the selection of the most suitable extraction and dispersion solvents. These two solvents greatly affect the sensitivity of the method. Secondly, since the DLLME method uses organic solvents for extraction, it may result in solvent residues in the extract. This may interfere with subsequent analytical results, especially in analyses with high sensitivity requirements.
-
The removal methods of 2,4,6-TCA generated in cork are one of the research hotspots in the field of wine safety and quality. However, few studies have been conducted on the methods for the elimination of 2,4,6-TCA in wine. With the development of analysis technology and control methods, research on the removal of wine odor substances has gradually developed. According to the source of 2,4,6-TCA, the control methods mainly include two ways. One is the prevention of the intrusion or formation of 2,4,6-TCA, and the other is the removal of 2,4,6-TCA. Some recommendations for reducing the risk of 2,4,6-TCA contamination in wine are shown in Fig. 6.
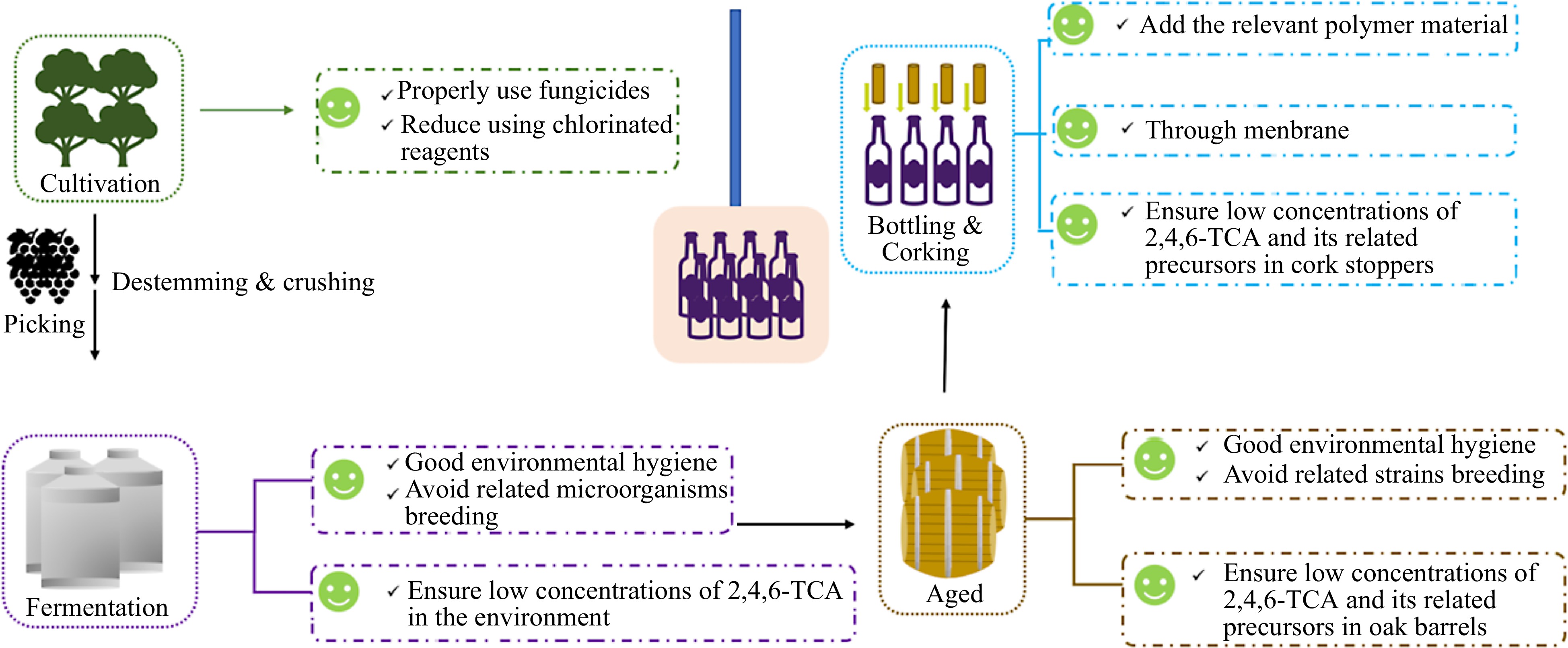
Figure 6.
Possible ways in which 2,4,6-TCA can contaminate wine, and recommendations for reducing the risk of 2,4,6-TCA contamination.
Removal of 2,4,6-TCA from contaminated wine
-
Consumers often realize that cork taint has occurred in wine. Because cork taint is still mainly caused by the cork. Therefore, to reduce the loss of business, most research still focuses on removing the relevant compounds from cork-tainted wine. Most remediation methods for cork-tainted wines focus on using a variety of materials, including polymer material and membrane filtration techniques.
Polymer material
-
Because of the unpleasant organoleptic effects of 2,4,6-TCA on wine and the growing body of research showing that 2,4,6-TCA does not originate only in cork, it is necessary to find an effective method of eliminating or minimizing 2,4,6-TCA with minimal impact on wine quality. In the past, it was common practice for small wineries to remediate TCA by blending slightly contaminated wines with uncontaminated wines to reduce the TCA concentration to sensory thresholds, but this method tended to contaminate large amounts of wine. As a result, researchers have also tried different methods to remove TCA from wine, with studies suggesting that aqueous suspensions of activated carbon from coconuts could eliminate 'corkiness' and synthetic aliphatic polymers (UHMWPE) could be used to effectively reduce 2,4,6-TCA concentrations in wine[83,84]. Molecularly imprinted polymers (MPPs) are synthetic materials with synthetic recognition sites that specifically bind to target molecules and have been shown to be effective in removing 2,4,6-TCA from wine[85]. The use of cork residue and cork powder as bio-sorbent is effective in the removal of pesticides and other pollutants from wastewater. Cosme et al. improved the adsorption performance of cork waste material and the addition of 0.25 g/L significantly reduced 2,4,6-TCA by 91%[84]. Valdés et al. tested sodium alginate, polyaniline emeraldine base (PANI-EB), polyaniline emeraldine salt (PANI-ES) and three generations of different cross-linked derivatives (G3, G4 and G5) of polyamides on 2,4,6-TCA and showed that their adsorption capacities on 2,4,6-TCA were all greater than 75% and did not affect the concentration of phenolics in wine, which has potential applications[83].
However, the addition of these new substances to the wine, whether new substances will be introduced, and whether these substances will cause quality safety issues remains to be explored. Therefore, the application of this method in practice needs to be further explored.
Membrane filtration techniques
-
The above-mentioned substances are often with low selectivity and may affect other compounds in the wine, thus affecting the quality of the wine. Therefore, researchers considered membrane filtration techniques with some selectivity. The depth filter sheet FIBRAFIX® TX-R, invented by the company Filtrox group (Zwingen, Switzerland), proved to be effective in removing 2,4,6-TCA and 2,4,6-TBA from wine[86]. However, the loss of esters and monoterpenes in the filtered wine and the high cost of the special filter sheet make its practical application a matter of consideration. González-Centeno et al. used alimentary film to adsorb 2,4,6-TCA from red wines and found that the removal rate was 81%−83%[79]. And it did not affect the total phenol and tannin content of the wine, as well as on the content of some volatile compounds, but may have a significant absorption effect on some esters, without affecting the fruitiness of the wine. Thus, it has a potential application prospect.
Control 2,4,6-TCA taint in wine from the source
-
Although there are two ways of 2,4,6-TCA formation, the main one is through O-methylation by microorganisms. Microorganisms are present in abundance and diversity, both in the vineyard, during fermentation, and in the environment in which fermentation and bottle storage take place. In particular, the microorganisms in the vineyard are often the key to the characterization of the wine. Therefore, it is impossible to prevent 2,4,6-TCA from forming in grapes and wine. 2,4,6-TCA can contaminate wine from cork stoppers or cellar environment. Thus, we can prevent 2,4,6-TCA from contaminating wine from these two sources.
Control contaminated cork stoppers
-
Cork stoppers are highly effective as wine sealers, allowing the wine to develop and age over time. However, cork taint was first discovered in cork stoppers, and now, cork stoppers are still considered to be the main source of 2,4,6-TCA in wine. The cork industry has tried to prevent, control, or even eradicate 2,4,6-TCA, but it is a tricky action. Thus, developing a rapid, operational and non-destructive method for the detection of 2,4,6-TCA in cork stoppers is pressing. By testing each cork stopper before use, contaminating wine can be radically avoided.
Secondly, developing an effective and economical technology to eliminate 2,4,6-TCA in cork is also important for prevention of the intrusion or formation of 2,4,6-TCA in wine, and reducing cost allowance. Electrochemical (EC) technology can control the chemical properties of water by electrolysis, thus creating favorable conditions for the reduction and oxidation of the removed targets. Since this method is renewable, and environmentally friendly without the use of chemical reagents, we can regulate the reaction rate by controlling the current intensity. Therefore, this method is gaining attention and has been studied for the removal of different contaminants from several matrices alone or in combination with other techniques. Guedes et al. applied the EC technique to the removal of 2,4,6-TCA from cork discs and found that the application of low-level direct current was able to remove 2,4,6-TCA from cork discs[87]. However, since cork discs are insulated, immersion of cork discs in a water bath is required for more efficient removal. And then their results showed that it can reduce 41% of the 2,4,6-TCA (2−5 ng/L) contaminated cork discs to 0.49 ng/L under optimal conditions and reducing 85% of the contaminated cork discs to 1.5 ng/L.
The activation of hydrogen peroxide is capable of generating a large number of oxidative radicals, like hydroxyl groups and/or single oxygen, that can react and destroy phenolic compounds. Because haloanisoles and halophenols are very similar in chemical structure. Therefore, Recio et al. investigated the catalytic degradation of 2,4,6-TCA in cork by molybdate ions under alkaline conditions with hydrogen peroxide as the oxidizing agent and found that it could reduce the 2,4,6-TCA content in cork by 86%[88]. In this regard, previous studies have also proposed the employment of heterogeneous photocatalysis to destroy 2,4,6-TCA during the storage of cork stoppers. Vlachos et al. used titanium dioxide as a photocatalyst to effectively remove 2,4,6-TCA from cork stoppers under a low-intensity near-UV radiation source[89].
The unique chemical reaction and energy transfer between gaseous plasma and water occur in the absence of any other chemicals, yet produces a product with remarkable instantaneous broad-spectrum biology activity known as plasma active water (PAW). Research showed it can inactivate plant-related pathogenic organisms and deactivation of bacteria and viruses, due to the presence of active ingredients such as ROS and RNS. Sainz-Garcia et al. used PAW generated during 5 min of plasma activation time in which contaminated corks were individually immersed for 3 h. Results show that 75.2% of 2,4,6-TCA was removed[90]. In addition, the reacting substance that plays a major role in the decomposition of 2,4,6-TCA, as well as other chloroanisole and chlorophenol molecules, was identified as OH·. The mechanism of OH· degradation of 2,4,6-TCA: firstly, demethylation is produced by a hydroxylation reaction, followed by an attack of the Cl atom by OH·.
Control contaminated environment (like air)
-
As we know wines may contain contaminant precursors before bottling and during storage due to contamination of the cell environment. To prevent contamination of wine during storage in the cellar, it is important to strictly control the concentration of chloroanisoles and their precursors in the cellar air. Fang et al. evaluated a non-thermal plasma air purification technology on removing two airborne haloanisole compounds, such as 2,4,6-TCA and 2,4,6-TBA. Laboratory test results showed that the non-thermal plasma air purification technology is effective in removing 2,4,6-TCA and 2,4,6-TBA and its single pass efficiency was higher than 82%. The field study showed effective reduction of airborne 2,4,6-TCA and 2,4,6-TBA in a wine cellar after 5-d operation of non-thermal plasma air purifiers[91]. The air purifiers tested in this study used close-coupled field technology (CCFT), which is generated by a controlled low-level non-thermal plasma with the addition of an electromagnetic field and a destructive cloud of supercharged electrons. When a compound is subjected to a closed-coupled field, the supercharged electrons may act on covalent or electrically charged bonds, separating them and causing molecular rupture.
Others
-
Many studies suggested that using chlorine-containing reagent can increase the risk of 2,4,6-TCA taint[42,43,71]. And TCA was originally found in corks that had been bleached with chlorine bleach[2]. Furthermore, to reduce 2,4,6-TCA taint and economic losses, strict prevention and control should be carried out. Firstly, the use of fungicides, insecticides, herbicides and other organic pesticides containing chlorophenols is strictly prohibited or minimized during the grape ripening period to reduce the contamination of 2,4,6-TCA at the source. Secondly, the use of chlorine-containing fungicides is strictly prohibited or minimized during the brewing process. Last but not least, keeping hygiene clean in winery and cellar can avoid related microorganisms breeding.
-
2,4,6-TCA resulting in cork taint is a devastating problem for the wine industry. 2,4,6-TCA is mainly generated by microbial O-methylation of chlorophenols. Using contaminated cork stoppers, environmental 2,4,6-TCA, and chlorinated reagents in the vineyard and winemaking contribute to TCA taint in wine. The sensory threshold for 2,4,6-TCA is extremely low, even 2,4,6-TCA at low concentrations in wine, it can impair wine quality. Accurately identifying and quantifying 2,4,6-TCA in wine, as well as cost-effective removing and controlling 2,4,6-TCA in wine, are extremely important to minimize wine industry losses. To have deeper perceptions of TCA taint, several important topics related to TCA taint are suggested to be further studied in future work.
First, the O-methylation of the 2,4,6-TCP precursor is the dominant pathway for the biosynthesis of 2,4,6-TCA, which is catalyzed by CPOMTs. There are few studies that have identified the characteristics of CPOMTs in water research. There is still a lack of research focusing on this problem on wine research. In future studies, it is believed that some advanced methods, such as metagenomics, macro-transcriptomics, and macro-proteomics, will be promising tools to reveal more comprehensive mechanism of O-methylation of chlorophenol precursor. Furthermore, the contributions of other multihalo-anisoles, such as 2,3,4,6-tetrachloroanisole, pentachloroanisole and 2,4,6-tribromoanisole to cork taint can also be further studies processes.
Second, microorganisms are in flux in the vineyard and the winemaking process. The community structure in the vineyard is different in different seasons or the wine at different stages of vinification. Therefore, it is meaningful to systematically screen for strains capable of producing cork-taint-related odors. Using data-driven analysis to evaluate the formation potential related to TCA can be useful to prevent the corresponding strains from colonizing vineyards and wineries, which can also solve TCA contamination of wine at the source (vineyard and winemaking process).
Most of the research regarding the removal of TCA focused on adsorption. In general, these materials often reach adsorption saturation, which greatly increases the cost of the winery. Investigating the mechanism of TCA adsorption and solving the current adsorption saturation problem of these materials so that they can be recycled is a future concern. A few studies have mentioned that the yeast cells can reduce TCA concentration in wine, it is worth exploring in depth to reduce haloanisole and halophenol through looking for more economical and green alternative materials without affecting the original quality of the wine and the improvement of control strategies.
-
The authors confirm contribution to the paper as follows: study conception and design: Zhan J, You Y, Huang W, Zhou H; data collection: Zhou H, Xie Y, Wu T, Wang X, Gao J, Tian B; analysis and interpretation of results: Zhou H; draft manuscript preparation: Zhan J, You Y, Zhou H. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
This work was supported by the Funded by Bureau of Culture and Tourism of Fangshan District, Beijing (The research on improving the flavour quality of Fangshan Wine).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhou H, Xie Y, Wu T, Wang X, Gao J, et al. 2024. Cork taint of wines: the formation, analysis, and control of 2,4,6- trichloroanisole. Food Innovation and Advances 3(2): 111−125 doi: 10.48130/fia-0024-0011
Cork taint of wines: the formation, analysis, and control of 2,4,6- trichloroanisole
- Received: 01 March 2024
- Revised: 22 April 2024
- Accepted: 28 April 2024
- Published online: 20 May 2024
Abstract: Cork taint has devastating effects on the aroma and quality of the wine, which can cause an annual loss of may be up to more than one billion dollars. There are many causes of cork taint, but 2,4,6-trichloroanisole (2,4,6-TCA) is a major contributor, giving the wine a wet-moldy smell. This study provided a comprehensive overview of the occurrence, detection, and control/remediation of 2,4,6-TCA. The occurrence and formation mechanisms of 2,4,6-TCA mainly include microbial O-methylation of chlorophenols and chlorination of anisole. The source of 2,4,6-TCA in wine is the cork or other woodworks, but it is also possible to contaminate wine from the environment. Due to the extremely low odor threshold concentration of 2,4,6-TCA, the effective sample pre-enrichment for instrument identification and quantification is more important. The control/remediation strategies of 2,4,6-TCA mainly include eliminating 2,4,6-TCA in cork and removing 2,4,6-TCA from wine by adsorption. Finally, the challenges and possible future research directions in this research field were discussed and proposed.
-
Key words:
- Wine /
- 2,4,6-trichloroanisole /
- Cork taint /
- Analysis methods /
- Remediation methods