-
Pork is the most abundant and consumed meat in the world[1], providing many essential nutrients for the human body, such as protein, amino acids, fat, minerals, and vitamins. However, with the improvement of people's living standards, people's requirements for the quality and flavor of pork are also increasing. Among the main commercial pig breeds, DLY is one of the major commercial pig breeds in our country, which is known for its fast growth rate and high lean meat rate, but its IMF (intramuscular fat) content is low and its meat quality is inferior[2]. Local breeds of pigs are known for their high IMF content and unique flavor. IMF is closely related to visual marbling and is one of the most critical quality attributes of pork[3]. Compared with DLY, local pigs in China have attracted attention due to their excellent meat quality. However, high-fat content is also more prone to lipid oxidation, which is one of the main limiting factors affecting the quality and consumer acceptability of meat and meat products, as it affects the nutritional value and sensory properties of meat, reducing the consumer’s purchasing desire[4,5]. So oxidative stability is crucial for the quality of fresh and processed meat[6]. Oxidative stability is usually related to endogenous antioxidant capacity, which can protect the matrix from reactive oxygen species and free radicals. It is usually determined by gene expression related to hypoxia response and energy metabolism[7].
Earlier scholars had studied the content of antioxidant compounds and oxidative stability from domestic[8−10] and wild animals[11,12]. The nonenzymatic antioxidant compounds include primarily vitamin E homologues (tocochromanols: tocopherols and tocotrienols)[13], vitamin C (L–ascorbic acid), carotenoids (carotenes and xanthophylls), and phenolic and polyphenolic compounds[14]. The enzymatic antioxidant systems mostly include superoxide dismutase, catalase, and glutathione peroxidase[15], which can have an effective impact on many oxidation levels as primary and secondary antioxidants (as scavengers, quenchers, inactivators, chelators, inhibitors, and reductants)[16].
Previously, most studies about the impact of lipid oxidation endogenous antioxidant factors in meat or meat products have focused on the changes in lipid antioxidant factors during storage or processing[17]. However, the differences in endogenous antioxidant factors and oxidative stability of pork in different varieties have been rarely discussed.
Ningxiang Pigs (NX) from the Institute of Subtropical Agricultural Ecology, Chinese Academy of Sciences, Changsha, Hunan Province, Rongchang Pigs (RC) from the Pig Breeding Institute of Chongqing Academy of Animal Husbandry Sciences, and Duroc × Wujin Pigs (DW) from the Laopu Family Breeding Base in Xuanwei, Yunnan Province (China), and Duroc × Landrace × Yorkshire (DLY) from Beijing in the same batch with the same feeding conditions, gender, and age were slaughtered at the slaughterhouse, and the outer ridges of the left half of the carcass gathered within 4 h and kept at −20 °C. The aim is to explore the variations in endogenous antioxidant factors and oxidative stability of pork across different varieties, with the intention of establishing a theoretical framework for enhanced regulation of lipid oxidation in pork while upholding its exceptional quality.
-
A total of 24, 6 month-old pigs, that meet the requirements to be put out, including six Duroc × Landrace × Yorkshire (DLY), six Ningxiang pigs (NX), six Rongchang pigs (RC), and six Duroc × Wujin pigs (DW) and live weight of 95−130 kg were selected. Following the period of growth, the pigs were slaughtered at a nearby slaughterhouse. The handling of all pigs adhered to the Regulations on Administration of Hog Slaughter and Good manufacturing practice for pig slaughter (GB/T 19479-2004) in China. The left longissimus muscle of each pig was removed from its carcasses and then subjected to vacuum packing, samples were then stored in the refrigerator until completely frozen and transported from the slaughterhouse cold chain to the laboratory for storage at −20 °C.
Determination of IMF content
-
The determination of IMF content is based on the Soxhlet extraction method in GB 5009.6-2016 'National Food Safety Standards - Determination of Fat in Foods'. Samples of pork dorsal muscles from different places are taken and dried in a 105 °C oven for 8 h to remove moisture. They are ground into powder using a mortar and placed in a receiving cup. After adding petroleum ether, they are extracted using a Soxhlet extractor. After the extraction is completed, the receiving cup is dried in a 105 °C oven for 1 h to remove petroleum ether, then placed in a dryer for 30 min to cool until constant temperature and weight, and then weighed. The fat content was calculated based on the difference before and after the extraction of the receiving cup.
Determination of fatty acid content
-
The determination of fatty acid content in pork refers to the external standard method in GB 5009.168-2016 'National Food Safety Standard - Determination of Fatty Acids in Foods'. The pork mince is freeze-dried into meat powder, and 0.500 g (± 0.005) of the freeze-dried meat powder is sequentially added to 5.0 mL of toluene and 6 mL of 10% acetyl chloride methanol solution. The extraction is carried out in a constant temperature water bath at 80 °C for 2 h. After extraction, the reaction solution is transferred to a 50 mL centrifuge tube and washed with 6% sodium carbonate solution. After centrifugation, the supernatant was taken for gas chromatography determination, and mixed fatty acid methyl esters were used as external standards for quantification.
Determination of lipid oxidation characterization value
-
To compare the degree of lipid oxidation in four pork varieties, Thiobarbital acid reactants (TBRAS), peroxide value (POV), conjugated diene (CD) and Lipid Peroxide (LPO) were selected as the lipid oxidation characterization indexes.
Determination of Thiobarbital acid reactants (TBRAS)
-
Referring to the method of Descalzo & Sancho[18], a 0.5 g cutting of an appropriate size of meat sample ws taken, 3 mL of thiobarbituric acid (TBA) solution and 15 mL of trichloroacetic acid (TCA) solution were added. The samples were then placed in a water bath at 90 °C for 40 min. When the system cools down, 5 mL of chloroform was added and centrifuged at 3,000 g for 5 min. Measure the absorbance value at 532 nm, calculate TBARS according to Eqn (1), and the result is expressed as the milligrams of malondialdehyde (MDA) per kilogram of meat sample.
$\rm TBRAS = A 532 \times 9.48 $ (1) In the formula, TBRAS − content of thiobarbituric acid reactants, mg MDA/kg; A532 − absorbance value of solution; 9.48 − constant coefficient.
Determination of peroxide value (POV)
-
The POV in the sample is based on the method in GB 5009.227-2016 National Food Safety Standards - Determination of peroxide value in food, with slight modifications. The pork minclets were taken, mixed with petroleum ether, and then soaked for 14 h. The filtrate was filtered through a funnel filled with anhydrous sodium sulfate, and the petroleum ether was dried by a rotary evaporator under reduced pressure. The remaining residue after evaporation was taken for determination.
Determination of conjugated diene (CD) content
-
Referring to the method of Kim et al.[19], an appropriate size of meat sample was cut, 0.5 g was then taken and 5 mL of deionized water added at 10,000 r/min. Samples were then homogenized for 20 s, 0.5 mL of the homogenate was taken, and 5 mL of a mixture of n-hexane and isopropanol (V/V = 3:1) added for 2 min for extraction, then centrifuged at 2,000 r/min for 10 min. The absorbance of the supernatant was then measured at 233 nm. The molar extinction coefficient of CD at this wavelength is 25.5 × 103 L/(mol·cm), expressed as the number of micromoles of CD per milligram of meat sample.
Determination of lipid peroxide (LPO) content
-
Sample preparation: Accurately weigh the pork samples from various sources and add 0.9% physiological saline in the ratio of weight (g) : volume (mL) = 1:9. Ice bath homogenization was performed at 10,000 r/min for 30 s/time, and repeated twice to produce 10% tissue homogenization. Centrifuge at 4 °C and 2,500 r/min for 10 min, and collect the supernatant to determine the content of lipid peroxides.
Determination principle: Under the condition of reacting at 45 °C for 60 min, one molecule of LPO reacts with two molecules of chromogenic reagents to produce a stable chromophore with a maximum absorption peak at 586 nm. The content of LPO in the test sample can be obtained by comparing standard curves or calculating through formulas. The measurement steps and calculation of lipid peroxide content in pork tissue are based on the instructions for the Lipid Peroxide Determination Kit (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China, No. A106-1).
Determination of antioxidant capacity characterization value
-
In this study, the antioxidant capacity of four varieties of pork was comprehensively evaluated by total antioxidant capacity (T-AOC), DPPH free radical scavenging power (DPPH) and Hydroxyl Radical Inhibition (OH•−), the total antioxidant capacity was discussed from two perspectives: Ferric Reducing Ability of Plasma (FRAP) and ABTS radical scavenging ability (ABTS).
The Sample pretreatment and determination method of T-AOC (FRAP), T-AOC (ABTS) DPPH free radical scavenging power and Hydroxyl Radical Inhibition (OH•−) are based on the instructions for the T-AOC (FRAP) assay kit (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China, No. A015-3-1), the total antioxidant capacity (ABTS) assay kit (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China, No. A015-2-1), the instructions of the DPPH free radical scavenging assay kit (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China, No. A153-1-1) and the hydroxyl radical inhibition assay kit (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China, No. A018-1-1) respectively.
Determination of endogenous antioxidant factor
-
The endogenous antioxidant factor was mainly determined by antioxidant oxidase(GSH-Px, SOD, CAT), total phenol content (TPC), vitamin C, and vitamin E content are four common substances with antioxidant activity in pork.
Extraction and activity determination of antioxidant enzymes
-
Principle and method for measuring glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and catalase (CAT) enzyme activity is based on the instructions for the GSH-Px enzyme assay kit (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China, No. A005-1-1); the SOD enzyme assay kit (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China, No. A001-3-2) and the CAT enzyme assay kit (Nanjing Jiancheng Biotechnology Co. Ltd. Nanjing, China, No. A007-1-1) respectively.
Determination of Total Phenol Content (TPC)
-
Referring to the method of Wootton-Beard et al.[20] with slight modifications, measure the total phenol content, the pork sample was ground and weighed 2.000 g (± 0.005). Eighteen ml of 0.9% physiological saline was added, and ice bath homogenization was performed at 10,000 r/min for 30 s/time. The homogenization was repeated twice to produce 10% tissue homogenization. At 4 °C, 3,000 r/min, centrifugation was performed for 10 min, and the supernatant was taken at 500 μL, then mixed with an equal volume of deionized water. Twenty percent sodium carbonate solution (W/V) was then added to Folin Ciocalteau reagent to 1 mol/L and mixed thoroughly. Under dark conditions, the mixture is kept in a 25 °C water bath for 1 h. The sample is then centrifuged at 3,000 g and 4 °C for 10 min to remove excess sodium carbonate solution. The absorbance value of the supernatant was measured at 760 nm (as a blank control), and a known concentration of gallic acid solution was used as the standard curve. The total phenolic concentration of the extract was expressed as milligrams of gallic acid (GAE) per gram of muscle.
Determination of vitamin C and vitamin E content
-
Sample pre-treatment and determination principle and method are based on the instructions for the Vitamin C Content Determination Kit (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China, NO.A009-1-1) and the Vitamin E Content Determination Kit (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China, A008-1-1).
Data processing and analysis
-
All experimental data were organized in Excel 2019 and statistical analysis was performed using SPSS 26.0 software (IBM, Chicago, IL, USA), Additionally, the data obtained were subjected to a one-way analysis of variance (ANOVA), and Duncan’s multiple comparison was used to determine the difference, the significance levels are defined at p < 0.05. Principal component analysis (PCA) through the correlation coefficient matrix method was applied for multivariate analysis using the 'OriginPro 2021'. Correlations between total antioxidant capacity, DPPH, OH•-, and antioxidant factors were determined by correlation analyses using Pearson’s linear correlation coefficient procedure.
-
The IMF content measurement results are shown in Table 1, the IMF content of RC was the highest, while the DLY was the lowest. The IMF content of NX and DW was between RC and DLY, as shown in Table 1, and there was a significant difference (p < 0.05). This result is consistent with previous research on the IMF content of local breeds and commercial pigs. Zhang et al.[2] found that the IMF content of local pigs in China is higher than that of commercial pigs. Zhang et al.[3] compared the differences in lipids and metabolites between Jianhe White Xiang pig and Large White pig, and the results showed that the IMF content of Jianhe White Xiang pig was 7.88%, significantly higher than that of Large White pig by 3.04%. The high-fat content also causes local pigs to be more prone to fat oxidation.
Table 1. Differences of fat and fatty acid content in different varieties of pork.
Item DLY NX RC DW FAT (g/100 g) 1.42 ± 0.32c 3.17 ± 0.56b 3.72 ± 0.30a 3.15 ± 0.27b SFA (g/100 g) 3.30 ± 0.34C 4.99 ± 0.46b 6.95 ± 0.50a 5.53 ± 0.35b MUFA (g/100 g) 3.69 ± 0.39a 5.47 ± 0.51b 6.30 ± 0.59b 6.92 ± 0.53b PUFA (g/100 g) 1.45 ± 0.09b 1.76 ± 0.09a 1.91 ± 0.08a 1.48 ± 0.05b FAs (g/100 g) 8.44 ± 0.80b 12.23 ± 1.03a 15.15 ± 1.11a 13.94 ± 0.91b DLY (Duroc × Landrace × Yorkshire pig); NX (Yorkshire × Ningxiang pig); RC (Rongchang pig); DW (Duroc × Wujin pig). The values are presented by means ± standard deviations. a, b mean values with various superscripts in a row were significantly different (p < 0.05). n = 6. The main step of lipid oxidation is the decomposition of the fatty acid into lower molecular weight compounds, and compared with saturated fatty acids, unsaturated fatty acids are more easily oxidized. Studies have shown that monounsaturated fatty acids (MUFA) are the main fatty acids (FAs) in Chinese native pig meat[21]. The results of determining the content of saturated fatty acids (SFA) in pigs from four different varieties showed in Table 1, that RC had the highest content of saturated fatty acids (SFA) and polyunsaturated fatty acids (PUFA); DW has the highest MUFA content (p < 0.05). Due to the significantly lower total fat content of DLY compared to the other three types of pigs, the content of each fatty acid in DLY is lower than that of the other three types. Rey et al.[22] also confirmed a significant increase in PUFAs content in pork with edible flaxseed oil. Fatty acid composition is one of the main contributors to pork flavor and is influenced by breed and genotype[23]. Highly saturated fatty acids (SFAs) are considered to have positive effects on stabilizing fat oxidation[24]. Local breed pigs have high fat and fatty acid content, which may lead to high oxidation degree and increased oxidation products.
Analysis of lipid oxidation characterization value
-
Thiobarbital acid reactants (TBRAS) are lipid oxidation indicators that affect consumer acceptance[25], calculated from a standard curve of malondialdehyde (MDA), and expressed as mg of MDA per kg sample. Due to the presence of unsaturated bonds, PUFAs are usually more prone to oxidation than SFAs, resulting in the production of different metabolites, which may have adverse effects on human health, shelf life, and meat quality[1]. MDA is the main secondary lipid oxidation product[26]. Compared with other secondary metabolites of lipid peroxidation, MDA is stable and abundant and is considered one of the most important markers of lipid peroxidation status[27,28]. Peroxide value (POV) is a parameter that reflects the degree of fat oxidation, and is a quantitative indicator of the product of double bonds in unsaturated fatty acids combined with oxygen in the air, indicating the degree of fat oxidation. A high POV value indicates a significant accumulation of intermediate products from fat oxidation, but these intermediate products will quickly undergo further oxidation reactions to generate small molecule substances as they accumulate[29]. Conjugated Dienes (CD) is the amount of conjugated diene contained in the oil was expressed as an index of lipid hydroperoxides in the extracted lipid fraction of the pork[19]. Lipid peroxidation (LPO) is the oxidation of polyunsaturated fatty acids (PUFAs) in biological systems and causes changes in the physical structure and characteristics of the cell membrane.
The determination results of lipid oxidation degree of four local pork varieties are shown in Table 2. There was no significant difference in POV, CD, and LPO, among the four local pork varieties. The content of TBRAS in DLY was significantly higher than that of the other three varieties (p < 0.05), indicating that the degree of fat oxidation of DLY was higher than that of the other three pork varieties and there was no significant difference in the content of TBRAS among the other three varieties. Previous studies have found that pig breed is one important factor of the MDA content of porcine Longissimus dorsi muscle during storage. The MDA contents of Large White pigs were significantly higher than Laiwu pigs (p < 0.01) when the muscles stored at 4 and −20 °C[30]. Hu el.[31] also confirmed this, and their results indicate that compared with the Yorkshire pigs, the Anqingliubai pig exhibited lower MDA concentration (p < 0.05). Previous studies have shown that the degree of unsaturated fatty acids is the main factor determining the amount of MDA formation. The results of lipid oxidation showed that although the local breeds of pigs have high fat and fatty acid content. However, the amount of oxidized products and the degree of oxidation are not much different from the DLY with lower fat content. This may be related to the differences in the antioxidant capacity of different varieties of pork due to different endogenous antioxidant factors, such as antioxidant enzyme activity, polyphenol content, and vitamin E content. It has been also observed that vitamin E and vitamin C delay the formation of MDA, during the oxidation of rat liver microsomes and phospholipid liposomes catalyzed by ferrous ions[32].
Table 2. Differences of lipid oxidation indices in different varieties of pork
Item DLY NX RC DW TBRAS (mg MDA/kg) 0.63 ± 0.03a 0.56 ± 0.01b 0.59 ± 0.05b 0.57 ± 0.02b POV (meq/kg) 0.16 ± 0.02a 0.14 ± 0.01a 0.14 ± 0.04a 0.17 ± 0.02a CD (mol/g) 1.52 ± 0.01a 1.43 ± 0.08a 1.50 ± 0.01a 1.51 ± 0.01a LPO (μmol/g prot) 0.14 ± 0.01a 0.14 ± 0.01a 0.17 ± 0.02a 0.16 ± 0.02a The values are presented by means ± standard deviations. The lowercase letterings a and b mean values with various superscripts in a row were significant differences (p < 0.05). n = 6. Analysis of antioxidant capacity characterization value
Total antioxidant capacity (T-AOC)
-
T-AOC is a comprehensive indicator of overall antioxidant capacity[33]. This study comprehensively evaluated its total antioxidant capacity (T-AOC) through two methods: iron ion reduction ability (FARP) and ABTS radical scavenging ability (ABTS). The FRAP test is a technique that measures the ability of antioxidants to reduce ferric ions (Fe3+) to ferrous ions (Fe2+)[34]. The foundation of the ABTS test is based on the measurement of the ability of antioxidants to reduce the previously generated cationic radical[35]. The measurement results as shown in Fig. 1, and NX had the highest FARP and ABTS radical scavenging ability (p < 0.05), while DLY had the lowest FARP, and there was no significant difference in ABTS radical scavenging ability among RC, DW, and DLY varieties( p > 0.05), as shown in Fig. 1b. This difference may be due to the different types and contents of antioxidant-active substances in different varieties of pork. There are research findings that state using up to 4% grapeseed oil in lamb diets may be a good method to improve meat antioxidant activity and fatty acid composition, without affecting animal production performance[33], which may be due to the fact that grape seeds contain a large number of substances with antioxidant activity, such as antioxidant enzymes, polyphenols and vitamin E, etc. Moreover, the pig breed is one important factor on the T-AOC of porcine Longissimus dorsi muscle. Hu et al.[31] confirmed the Anqingliubai pigs exhibited higher T-AOC compared with the Yorkshire pigs (p < 0.05). Chen et al.[36] also confirmed that meat had more total antioxidant capacity in Chinese native and breed pigs than DLY (p < 0.05).
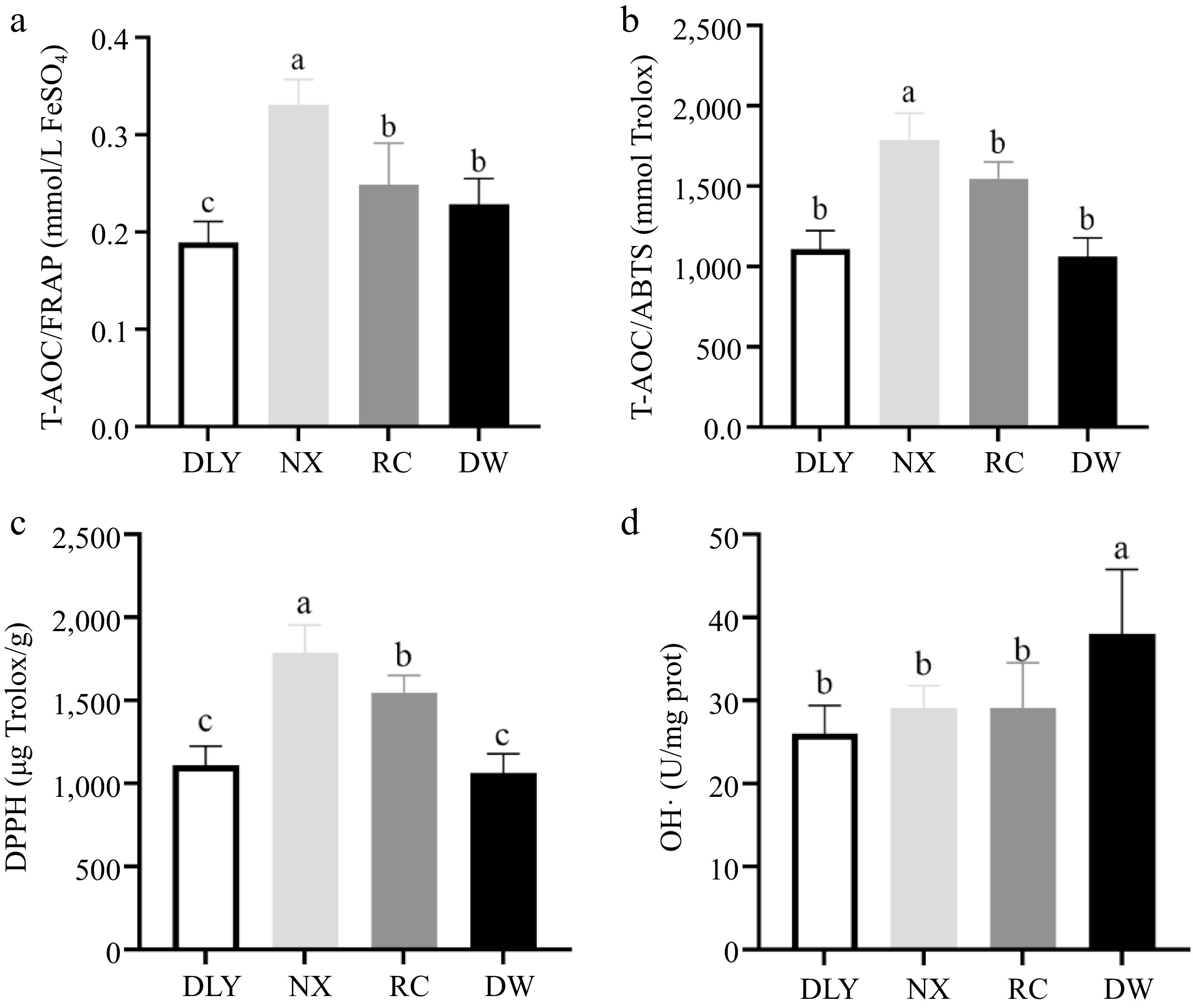
Figure 1.
Comparison of antioxidant capacity characterization value of four varieties of pork. (a) Iron ion reduction ability (FARP); (b) ABTS radical scavenging ability (ABTS); (c) Comparison of DPPH among four varieties of pork; (d) Comparison of OH•- among four varieties of pork. Different letters indicate significant differences between pork samples of different breeds (p < 0.05). n = 6.
Free radical scavenging ability (DPPH)
-
DPPH can reflect the ability of the muscle antioxidant system to scavenge free radicals, this ability may come from vitamin C, polyphenols and some antioxidant activity of reducing substances[18]. The DPPH test is based on the measurement of the reducing capacity of antioxidants against the free radical DPPH. This measurement is generally carried out through the determination of the decrease in the absorbance, which is currently a technique judged a standard for the in vitro determination of antioxidants that is extensively employed for the evaluation of free radical scavenging potentials of distinct compounds[35]. The results of DPPH free radical scavenging ability measurement of four local pork samples are shown in Fig. 1c, the NX had the highest DPPH free radical scavenging ability, significantly higher than RC, while DW and DLY had the lowest DPPH free radical scavenging ability, significantly lower than RC (p < 0.05).
Hydroxyl radical inhibition ability (OH•−)
-
Hydroxyl radical plays a prominent role in the oxidation of protein, lipid, and nucleic acid in vivo because the oxidization ability of hydroxyl radicals is the highest among reactive oxygen species (ROS). Moreover, lipid peroxyl radicals (LOO•) produced by the oxidation of the lipid bilayer of the cell membrane by hydroxyl radical triggers a lipid peroxidation chain reaction and damages cell membranes. The inhibitory ability of hydroxyl radical (OH•−) can represent the antioxidant ability[37]. The measurement results of the inhibitory ability of hydroxyl radical (OH•−) of four local pork varieties showed that DW had the highest hydroxyl radical inhibition ability, significantly higher than NX, RC, and DLY (p < 0.05). There was no difference in hydroxyl radical inhibition ability among the other three varieties (p > 0.05), as shown in Fig 1d. This showed that DW had stronger ability to inhibit hydroxyl radical oxidation of membrane lipid bilayer, generate lipid peroxidation free radical and trigger lipid peroxidation chain reaction process than the other three varieties.
The above results indicated that there were differences in T-AOC/DPPH and inhibitory ability of hydroxyl radical (OH•−) of different pork varieties, which might be attributed to the differences in endogenous antioxidant factors and the types and contents of antioxidant active substances contained in different pork varieties, such as antioxidant enzymes, polyphenols, vitamin C and vitamin E.
Analysis of endogenous antioxidant factors
Antioxidant enzyme activities
-
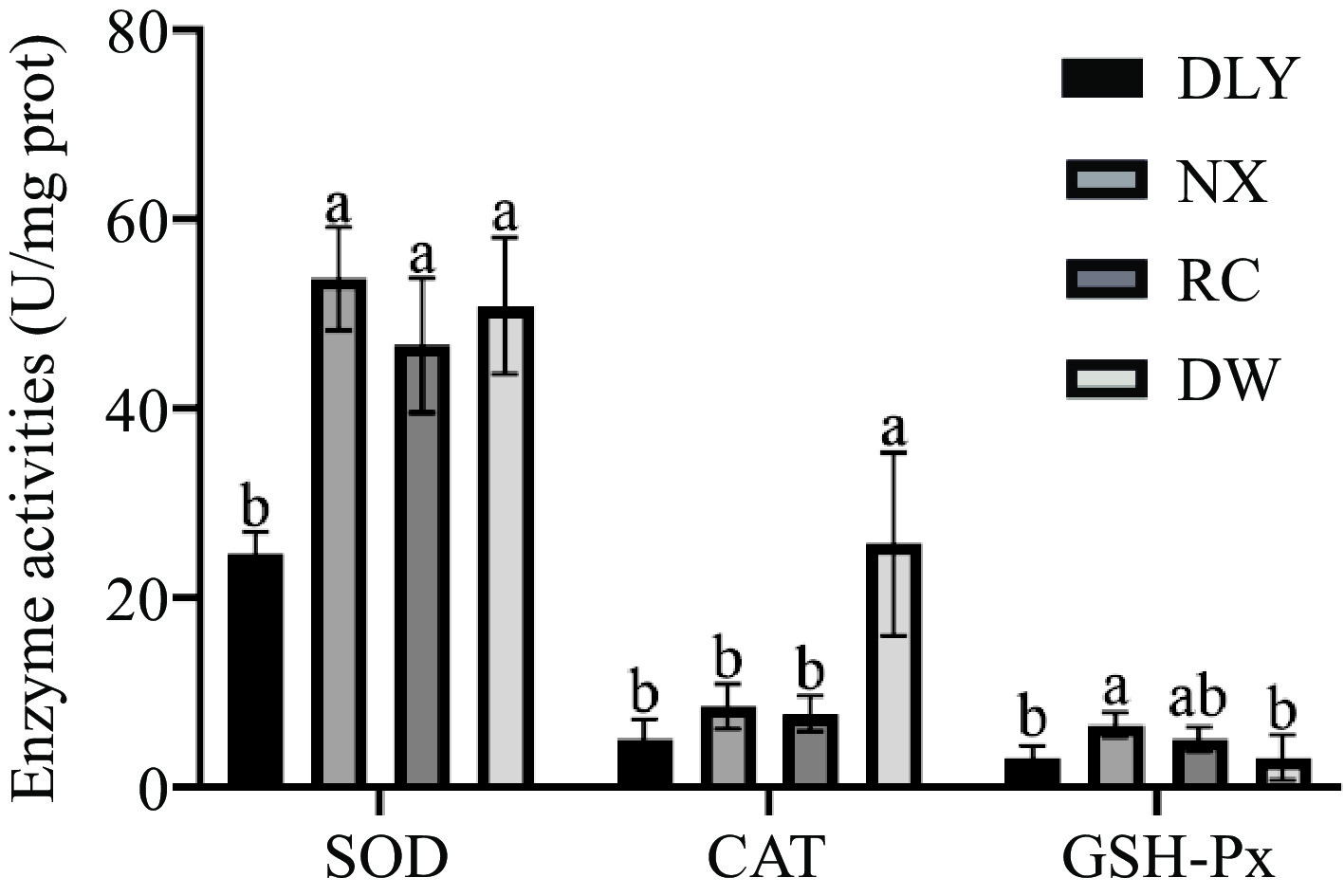
Antioxidant enzymes are considered as important protectors in muscles to prevent lipid oxidation. The endogenous antioxidant enzymes in muscles mainly include superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). SOD is the first line of defense of the antioxidant system[7]. CAT is a peroxidase that catalyzes the decomposition of hydrogen peroxide into water and oxygen[38]. GSH-Px inhibits further oxidative damage[39]. The results of endogenous antioxidant enzyme activity measurements of four breeds of pork are shown in Fig. 2, that among the four types of local pork, the SOD enzyme, and GSH-Px enzyme activities of NX were the highest; DW have the highest CAT enzyme activity; The activity of SOD and CAT enzymes in DLY is the lowest; The activity of GSH-Px enzyme in DW pigs was the lowest, but there was no significant difference compared to DLY (p < 0.05). Differences in the activity of the three antioxidant enzymes may lead to differences in the antioxidant capacity of the four varieties pork. Studies have shown that catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) can effectively delay lipid oxidation in beef[40]. Chen et al.[30] found in their research that the Laiwu pig as a Chinese indigenous pig breed showed strong SOD, CAT and GSH-Px activity, and it suggested that Laiwu pig can more effectively inhibit lipid oxidation and have a long shelf-life after slaughter compared to the Large White pig.
Total phenol content (TPC)
-
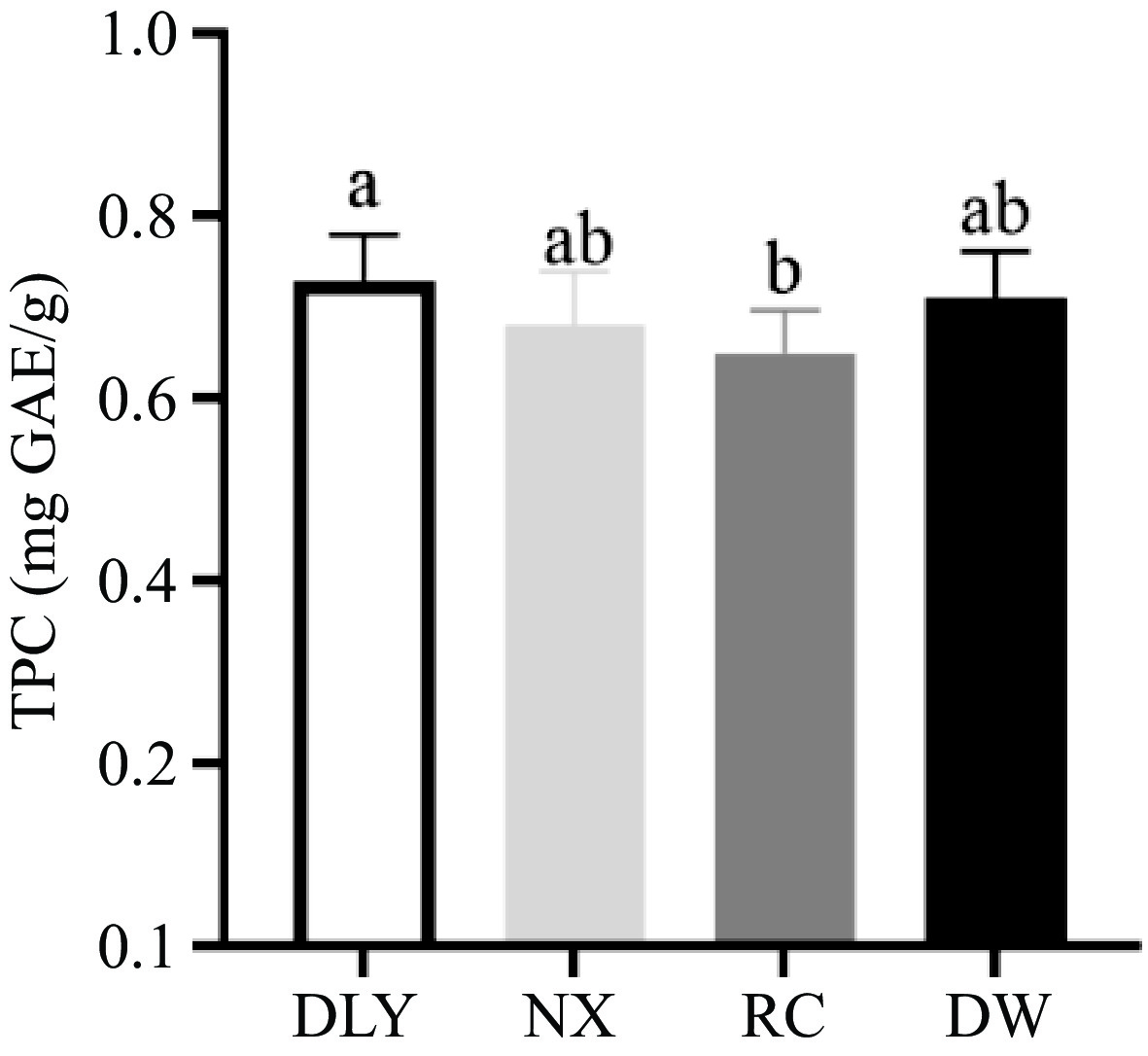
Polyphenols have been shown to have various antioxidant activities, including scavenging free radicals, inhibiting enzymes involved in free radical production, and chelating metal ions[41]. The Folin Ciocalteu trial can be considered a method to determine the antioxidant capacity by electron transfer, and high content of phenolic compounds in foods have been associated with high antioxidant capacity. From the results shown in Fig. 3, the total phenol content of RC is the lowest, but there is no significant difference from the total phenol content of NX and DW, and the total phenol content of DLY is the highest (p < 0.05) but there was no significant difference with NX and DW content. Yusuf et al.[42] found that there was no difference in the percentage of fat, water, protein, and ash components in goat muscle in the feed supplemented with different parts of Andrographis paniculata, but there were differences in polyphenol content, which lead to differences in antioxidant capacity. This is consistent with the observation of Qwele et al.[43], where eating spicy wood leaves increased the total phenol content in goat meat, and also improved antioxidant capacity. These studies indicated that exogenous TPC could affect the antioxidant capacity of meat, but the endogenous TPC in fresh meat had a relatively limited effect on the antioxidant capacity due to their low content. To our knowledge, only limited information about the phenolic content in pig meat have been previously reported.
Vitamin C and Vitamin E content
-
Vitamins C and E function as water-soluble and lipid-soluble chain-breaking antioxidants, respectively, and protect lipids, proteins, and membranes from oxidative damage. Vitamin C scavenges oxygen radicals in the aqueous phase, whereas vitamin E scavenges oxygen radicals within the membranes. This interaction between vitamin C and vitamin E radicals can take place not only in homogeneous solutions but also in liposomal membrane systems[44]. It has been also observed that vitamins E and C delay the formation of MDA, during the oxidation of rat liver microsomes and phospholipid liposomes catalyzed by ferrous ion[32].
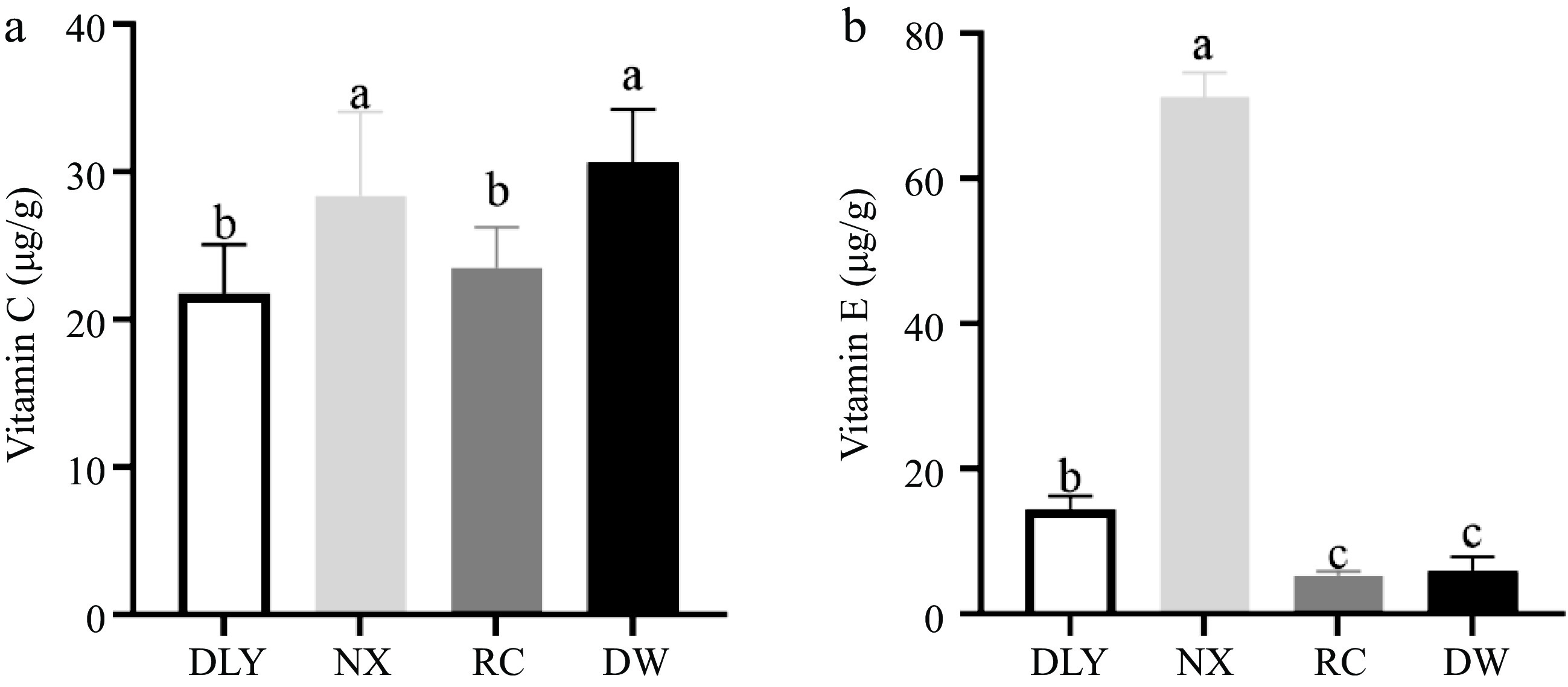
The results of vitamin C determination for four types of local pork were shown in Fig. 4a, the vitamin C content of DW was the highest, significantly higher than RC and DLY, but there was no significant difference compared to NX content. The NX had the highest vitamin E content, significantly higher than DLY, while RC and DW had the lowest vitamin E content, significantly lower than DLY as shown in Fig. 4b (p < 0.05). The difference in vitamin C and vitamin E content in the four varieties of pork may affect the difference in their antioxidant capacity. Descalzo & Sancho[18] found that vitamin E in animal tissues affects the stability of lipids during the storage of meat. It is capable of quenching free radicals and thus protects phospholipids and cholesterol against oxidation, and a higher intake of natural antioxidants can result in transferring these molecules to animal tissues with a consequent increase of total antioxidant capacity[18]. The inhibitory effect of vitamin E on lipid peroxidation is documented based on in vivo experiments, Burdeos et al.[45] reported that the daily intake of tocotorienol-rich rice bran oil (10 mg of rice bran oil containing 3.14 mg α-tocotrie-nol, 5.04 mg γ-tocotrienol, 0.04 mg δ-tocotrienol, 0.19 mg α-TocH, 0.21 mg γ-TocH, and 0.20 mg δ-TocH by oral gavage using 200 μL corn oil as a vehicle) for 3 weeks decreased levels of plasma phospholipid hydroperoxide (PLOOH) in rats. This indicates that vitamin E can effectively enhance antioxidant capacity.
Correlations between antioxidant capacity and antioxidant factors
-
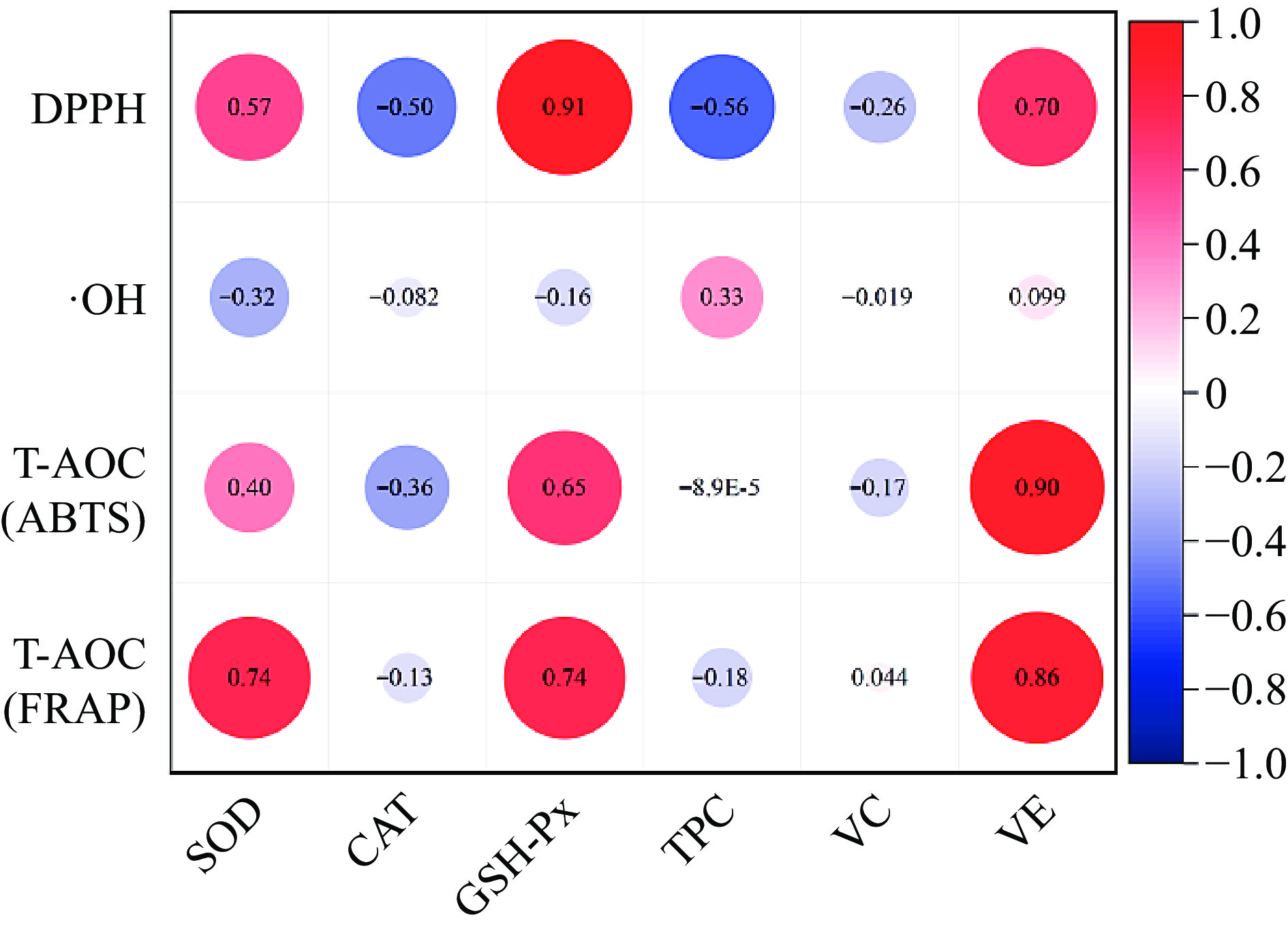
To evaluate the relationship between antioxidant performance and endogenous antioxidant factors of four pig breeds, antioxidant capacity was used as the Y variable and endogenous antioxidant factors as the X variable for Pearson correlation analysis. The results are shown in Fig. 5, and show that the T-AOC and DPPH free radical scavenging ability of the four pig breeds were positively correlated with SOD and GSH-Px activity, vitamin E content, and DPPH had the strongest correlation with GSH-Px activity, with a correlation coefficient of 0.91, The correlation between T-AOC and vitamin E content is strongest, with T-AOC (ABTS) and T-AOC (FARP) being 0.9 and 0.86, respectively. OH•− inhibitory capacity is positively correlated with TPC content, with a correlation coefficient of 0.33. It can be seen that the antioxidant factors that have significant effects on the antioxidant performance of the four varieties of pork are SOD, GSH-Px activity, vitamin E, and TPC content.
Antioxidant capacity analyses show NX had the highest FARP, ABTS radical scavenging ability, and DPPH, DW had the highest Hydroxyl Radical Inhibition Ability. Antioxidant factors analyses NX had the highest vitamin E content, the highest SOD activity and the highest GSH-PX activity, which may be the main factors to enhance the antioxidant capacity of NX. DW had the highest OH•−, although its TPC content was not the highest, there was no significant difference between it and DLY with the highest content. DLY had the lowest FARP among the four varieties, and ABTS and DPPH are significantly lower than NX, meanwhile, SOD and GSH-Px enzyme activity are the lowest, it’s vitamin E content is also significantly lower than NX. Which is consistent with the results of correlation analysis.
-
The oxidative stability of different varieties of pork has significant differences. Compared with DLY, the local pork varieties have higher fat and fatty acid content, but there is no significant difference in their content of lipid oxidation products compared to DLY, which maybe due to their strong antioxidant properties. Owing to the difference in endogenous antioxidant factors, NX pigs have the best antioxidant stability and lowest content of lipid oxidation products compared with DLY, RC, and DW, and the antioxidant factors that have a significant impact on the antioxidant performance of the four breeds pig meat are SOD, GSH-Px activity, vitamin E, and TPC content. Through this experiment, we can learn more about the differences in antioxidant stability of different varieties of pork, and provide a theoretical basis for regulating lipid oxidation of different varieties of pork, to retain the superior quality of local varieties of pork and provide basic data for the development of pork products.
-
The authors confirm contribution to the paper as follows: conceptualization, resources, visualization: Huang X, Tang X; methodology: Huang X, Tang X, Zhao Y, Zhang Y; software: Huang X, Zhao Y, Liu H; validation: Tang X, Zhang Y, Zhao Y; formal analysis, investigation, data curation: Huang X; original draft preparation, review and editing: Tang X, Huang X, Liu H; Supervision: Liu H, Zhang Y; project administration: Tang X, Huang X; funding acquisition: Tang X. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during the current study are available from the corresponding author on reasonable request.
This research was funded by the National Natural Science Foundation of China, Grant No. 32172152; the National Key R&D Program of China, Grant No. 2022YFD1601902, the China Agriculture Research System, Grant No. CARS-35 and the Agricultural Science and Technology Innovation Program of CAAS.
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Xinyuan Huang, Hui Liu
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Huang X, Liu H, Tang X, Zhang Y, Zhao Y. 2024. Exploration of different pork varieties affecting its lipid oxidation stability. Food Materials Research 4: e017 doi: 10.48130/fmr-0024-0008
Exploration of different pork varieties affecting its lipid oxidation stability
- Received: 08 December 2023
- Revised: 21 March 2024
- Accepted: 07 April 2024
- Published online: 19 June 2024
Abstract: The lipid oxidation degree of different pork varieties was different, which may be due to varying levels of antioxidant stability. The purpose of this research was to explore the endogenous antioxidant factors and disparities in oxidative stability between various pork varieties. According to the single-factor experimental design, six pigs from each of the Duroc × Landrace × Yorkshire (DLY), Ningxiang pig (NX), and Rongchang pigs (RC) and were selected, and the longissimus dorsi muscle was used as the experimental sample to determine the fat and fatty acid content, lipid oxidation characterization value, antioxidant capacity and endogenous antioxidant factors of the four varieties pork. The results show that the RC exhibited the highest total fat and fatty acid content, whereas the DLY displayed the lowest levels (p < 0.05). The Thiobarbital acid reactants (TBRAS) of DLY were significantly higher than the other three varieties, and there was no significant difference in peroxide value (POV), conjugated diene (CD), and lipid peroxide (LPO), among the four pork varieties. NX exhibited the highest level of comprehensive antioxidant activity, while Duroc × Landrace × Yorkshire (DLY) displayed the lowest level of comprehensive antioxidant activity (p < 0.05). The results suggested that the antioxidant stability of the four varieties of pork was different. Compared with DLY, RC, and DW, NX showed the best antioxidant stability and the lowest lipid oxidation degree. The activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), vitamin E, and total phenol content (TPC) were significantly correlated with antioxidant capacity.
-
Key words:
- Varieties /
- Pork /
- Lipid oxidation /
- Fatty acids