-
Starting in the early 2000s, China has experienced rapid growth as an emerging wine market. It has now established itself as the world's second-largest grape-growing country in terms of vineyard surface area. Furthermore, China has also secured its position as the sixth-biggest wine producer globally and the fifth-most significant wine consumer in terms of volume[1]. The Ningxia Hui autonomous region, known for its reputation as the highest quality wine-producing area in China, is considered one of the country's most promising wine regions. The region's arid or semiarid climate, combined with ample sunlight and warmth, thanks to the Yellow River, provides ideal conditions for grape cultivation. Wineries in the Ningxia Hui autonomous region are renowned as the foremost representatives of elite Chinese wineries. All wines produced in this region originate from grapes grown in their vineyards, adhering to strict quality requirements, and have gained a well-deserved international reputation for excellence. Notably, in 2011, Helan Mountain's East Foothill in the Ningxia Hui Autonomous Region received protected geographic indication status in China. Subsequently, in 2012, it became the first provincial wine region in China to be accepted as an official observer by the International Organisation of Vine and Wine (OIV)[2]. The wine produced in the Helan Mountain East Region of Ningxia, China, is one of the first Agricultural and Food Geographical Indications. Starting in 2020, this wine will be protected in the European Union[3].
Marselan, a hybrid variety of Cabernet Sauvignon and Grenache was introduced to China in 2001 by the French National Institute for Agricultural Research (INRA). Over the last 15 years, Marselan has spread widely across China, in contrast to its lesser cultivation in France. The wines produced from Marselan grapes possess a strong and elegant structure, making them highly suitable for the preferences of Chinese consumers. As a result, many wineries in the Ningxia Hui Autonomous Region have made Marselan wines their main product[4]. Wine is a complex beverage that is influenced by various natural and anthropogenic factors throughout the wine-making process. These factors include soil, climate, agrochemicals, and human intervention. While there is an abundance of research available on wine production, limited research has been conducted specifically on local wines in the Eastern Foot of Helan Mountain. This research gap is of significant importance for the management and quality improvement of Chinese local wines.
Ion mobility spectrometry (IMS) is a rapid analytical technique used to detect trace gases and characterize chemical ionic substances. It achieves this through the gas-phase separation of ionized molecules under an electric field at ambient pressure. In recent years, IMS has gained increasing popularity in the field of food-omics due to its numerous advantages. These advantages include ultra-high analytical speed, simplicity, easy operation, time efficiency, relatively low cost, and the absence of sample preparation steps. As a result, IMS is now being applied more frequently in various areas of food analysis, such as food composition and nutrition, food authentication, detection of food adulteration, food process control, and chemical food safety[5,6]. The orthogonal hyphenation of gas chromatography (GC) and IMS has greatly improved the resolution of complex food matrices when using GC-IMS, particularly in the analysis of wines[7].
The objective of this study was to investigate the changes in the physicochemical properties of Marselan wine during the winemaking process, with a focus on the total phenolic and flavonoids content, antioxidant activity, and volatile profile using the GC-IMS method. The findings of this research are anticipated to make a valuable contribution to the theoretical framework for evaluating the authenticity and characterizing Ningxia Marselan wine. Moreover, it is expected that these results will aid in the formulation of regulations and legislation pertaining to Ningxia Marselan wine in China.
-
All the grapes used to produce Marselan wines, grow in the Xiban vineyard (106.31463° E and 38.509541° N) situated in Helan Mountain's East Foothill of Ningxia Hui Autonomous Region in China.
Chemicals and reagents
-
Folin-Ciocalteau reagent, (±)-6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,20-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ), anhydrous methanol, sodium nitrite, and sodium carbonate anhydrous were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Reference standards of (+)-catechin, gallic acid, and the internal standard (IS) 4-methyl-2-pentanol were supplied by Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). The purity of the above references was higher than 98%. Ultrapure water (18.2 MΩ cm) was prepared by a Milli-Q system (Millipore, Bedford, MA, USA).
Preparation of Marselan juices and wines
-
Stage 1−Juice processing: Grapes at the fully mature stage are harvested and crushed, and potassium metabisulfite (5 mg/L of SO2) was evenly spread during the crushing process. The obtained must is transferred into stainless steel tanks. Stage 2−Alcoholic fermentation: Propagated Saccharomyces cerevisiae ES488 (Enartis, Italy) are added to the fresh must, and alcoholic fermentation takes place, after the process is finished, it is kept in the tanks for 7 d for traditional maceration to improve color properties and phenolics content. Stage 3−Malolactic fermentation: When the pomace is fully concentrated at the bottom of the tanks, the wine is transferred to another tank for separation from these residues. Oenococcus oeni VP41 (Lallemand Inc., France) is inoculated and malic acid begins to convert into lactic acid. Stage 4−Wine stabilization: After malolactic fermentation, potassium metabisulfite is re-added (35 mg/L of SO2), and then transferred to oak barrels for stabilization, this process usually takes 6-24 months. A total of four batches of samples during the production process of Marselan wine were collected in this study.
Total polyphenols and flavonoid content determination
-
Total polyphenols were determined on 0.5 mL diluted wine sample using the Folin-Ciocalteu method[8], using gallic acid as a reference compound, and expressed as milligrams of gallic acid equivalents per liter of wine. The total flavonoid content was measured on 0.05 mL of wine sample by a colorimetric method previously described[9]. Results are calculated from the calibration curve obtained with catechin, as milligrams of catechin equivalents per liter of wine.
ABTS free-radical scavenging assay
-
The antioxidative activity was determined using the ABTS·+ assay[10]. Briefly, the ABTS·+ radical was prepared from a mixture of 88 μL of potassium persulfate (140 mmol/L) with 5 mL of the ABTS·+ solution (7 mmol/L). The reaction was kept at room temperature under the absence of light for 16 h. Sixty μL samples were mixed with 3 mL of ABTS·+ solution with measured absorption of 0.700 ± 0.200 at 734 nm. After 6 min reaction, the absorbance of samples were measured with a spectrophotometer at 734 nm. Each sample was tested in triplicate. The data were expressed as mmol Trolox equivalent of antioxidative capacity per liter of the wine sample (mmol TE/L). Calibration curves, in the range 64.16−1,020.20 μmol TE/L, showed good linearity (R2 ≥ 0.99).
FRAP free-radical scavenging assay
-
The FRAP assay was conducted according to a previous study[11]. The FRAP reagent was freshly prepared and mixed with 10 mM/L TPTZ solution prepared in 20 mM/L FeCl3·6H2O solution, 40 mM/L HCl, and 300 mM/L acetate buffer (pH 3.6) (1:1:10; v:v:v). Ten ml of diluted sample was mixed with 1.8 ml of FRAP reagent and incubated at 37 °C for 30 min. The absorbance was determined at 593 nm and the results were reported as mM Fe (II) equivalent per liter of the wine sample. The samples were analyzed and calculated by a calibration curve of ferrous sulphate (0.15−2.00 mM/mL) for quantification.
HS-GC-IMS analysis
-
The volatile compounds were analyzed on a GC-IMS instrument (FlavourSpec, GAS, Dortmund, Germany) equipped with an autosampler (Hanon Auto SPE 100, Shandong, China) for headspace analysis. One mL of each wine was sampled in 20 mL headspace vials (CNW Technologies, Germany) with 20 μL of 4-methyl-2-pentanol (20 mg/L) ppm as internal standard, incubated at 60 °C and continuously shaken at 500 rpm for 10 min. One hundred μL of headspace sample was automatically loaded into the injector in splitless mode through a syringe heated to 65 °C. The analytes were separated on a MxtWAX capillary column (30 m × 0.53 mm, 1.0 μm) from Restek (Bellefonte, Pennsylvania, USA) at a constant temperature of 60 °C and then ionized in the IMS instrument (FlavourSpec®, Gesellschaft für Analytische Sensorsysteme mbH, Dortmund, Germany) at 45 °C. High purity nitrogen gas (99.999%) was used as the carrier gas at 150 mL/min, and drift gas at 2 ml/min for 0−2.0 min, then increased to 100 mL/min from 2.0 to 20 min, and kept at 100 mL/min for 10 min. Ketones C4−C9 (Sigma Aldrich, St. Louis, MO, USA) were used as an external standard to determine the retention index (RI) of volatile compounds. Analyte identification was performed using a Laboratory Analytical Viewer (LAV) 2.2.1 (GAS, Dortmund, Germany) by comparing RI and the drift time of the standard in the GC-IMS Library.
Statistical data analysis
-
All samples were prepared in duplicate and tested at least six times, and the results were expressed as mean ± standard error (n = 4) and the level of statistical significance (p < 0.05) was analyzed by using Tukey's range test using SPSS 18.0 software (SPSS Inc., IL, USA). The principal component analysis (PCA) was performed using the LAV software in-built 'Dynamic PCA' plug-in to model patterns of aroma volatiles. Orthogonal partial least-square discriminant analysis (OPLS-DA) in SIMCA-P 14.1 software (Umetrics, Umeă, Sweden) was used to analyze the different volatile organic compounds in the different fermentation stages.
-
The results of the changes in the antioxidant activity of Marselan wines during the entire brewing process are listed in Table 1. It can be seen that the contents of flavonoids and polyphenols showed an increasing trend during the brewing process of Marselan wine, which range from 315.71−1,498 mg CE/L and 1,083.93−3,370.92 mg GAE/L, respectively. It was observed that the content increased rapidly in the alcoholic fermentation stage, but slowly in the subsequent fermentation stage. This indicated that the formation of flavonoid and phenolic substances in wine mainly concentrated in the alcoholic fermentation stage, which is consistent with previous reports. This is mainly because during the alcoholic fermentation of grapes, impregnation occurred to extract these compounds[12]. The antioxidant activities of Marselan wine samples at different fermentation stages were detected by FRAP and ABTS methods[11]. The results showed that the ferric reduction capacity and ABST·+ free radical scavenging capacity of the fermented Marselan wines were 2.4 and 1.5 times higher than the sample from the juice processing stage, respectively, indicating that the fermented Marselan wine had higher antioxidant activity. A large number of previous studies have suggested that there is a close correlation between antioxidant activity and the content of polyphenols and flavonoids[13−15]. Previous studies have reported that Marselan wine has the highest total phenol and anthocyanin content compared to the wine of Tannat, Cabernet Sauvignon, Merlot, Cabernet Franc, and Syrah[13]. Polyphenols and flavonoids play an important role in improving human immunity. Therefore, Marselan wines are popular because of their high phenolic and flavonoid content and high antioxidant capacity.
Table 1. GC-IMS integration parameters of volatile compounds in Marselan wine at different fermentation stages.
No. Compounds Formula RI* Rt
[sec]**Dt
[RIPrel]***Identification
approachConcentration (μg/mL) (n = 4) Stage 1 Stage 2 Stage 3 Stage 4 Aldehydes 5 Furfural C5H4O2 1513.1 941.943 1.08702 RI, DT, IS 89.10 ± 4.05c 69.98 ± 3.22c 352.16 ± 39.06b 706.30 ± 58.22a 6 Furfural dimer C5H4O2 1516.6 948.77 1.33299 RI, DT, IS 22.08 ± 0.69b 18.68 ± 2.59c 23.73 ± 2.69b 53.39 ± 9.42a 12 (E)-2-hexenal C6H10O 1223.1 426.758 1.18076 RI, DT, IS 158.17 ± 7.26a 47.57 ± 2.51b 39.00 ± 2.06c 43.52 ± 4.63bc 17 (E)-2-pentenal C5H8O 1129.2 333.392 1.1074 RI, DT, IS 23.00 ± 4.56a 16.42 ± 1.69c 18.82 ± 0.27b 18.81 ± 0.55b 19 Heptanal C7H14O 1194.2 390.299 1.33002 RI, DT, IS 17.28 ± 2.25a 10.22 ± 0.59c 14.50 ± 8.84b 9.11 ± 1.06c 22 Hexanal C6H12O 1094.6 304.324 1.25538 RI, DT, IS 803.11 ± 7.47c 1631.34 ± 19.63a 1511.11 ± 26.91b 1526.53 ± 8.12b 23 Hexanal dimer C6H12O 1093.9 303.915 1.56442 RI, DT, IS 588.85 ± 7.96a 93.75 ± 4.67b 92.93 ± 3.13b 95.49 ± 2.50b 29 3-Methylbutanal C5H10O 914.1 226.776 1.40351 RI, DT, IS 227.86 ± 6.39a 33.32 ± 2.59b 22.36 ± 1.18c 21.94 ± 1.73c 33 Dimethyl sulfide C2H6S 797.1 193.431 0.95905 RI, DT, IS 120.07 ± 4.40c 87.a02 ± 3.82d 246.81 ± 5.62b 257.18 ± 3.04a 49 2-Methylpropanal C4H8O 828.3 202.324 1.28294 RI, DT, IS 150.49 ± 7.13a 27.08 ± 1.48b 19.36 ± 1.10c 19.69 ± 0.92c Ketones 45 3-Hydroxy-2-butanone C4H8O2 1293.5 515.501 1.20934 RI, DT, IS 33.20 ± 3.83c 97.93 ± 8.72b 163.20 ± 21.62a 143.51 ± 21.48a 46 Acetone C3H6O 836.4 204.638 1.11191 RI, DT, IS 185.75 ± 8.16c 320.43 ± 12.32b 430.74 ± 3.98a 446.58 ± 10.41a Organic acid 3 Acetic acid C2H4O2 1527.2 969.252 1.05013 RI, DT, IS 674.66 ± 46.30d 3602.39 ± 30.87c 4536.02 ± 138.86a 4092.30 ± 40.33b 4 Acetic acid dimer C2H4O2 1527.2 969.252 1.15554 RI, DT, IS 45.25 ± 3.89c 312.16 ± 19.39b 625.79 ± 78.12a 538.35 ± 56.38a Alcohols 8 1-Hexanol C6H14O 1365.1 653.825 1.32772 RI, DT, IS 1647.65 ± 28.94a 886.33 ± 32.96b 740.73 ± 44.25c 730.80 ± 21.58c 9 1-Hexanol dimer C6H14O 1365.8 655.191 1.64044 RI, DT, IS 378.42 ± 20.44a 332.65 ± 25.76a 215.78 ± 21.04b 200.14 ± 28.34b 13 3-Methyl-1-butanol C5H12O 1213.3 414.364 1.24294 RI, DT, IS 691.86 ± 9.95c 870.41 ± 22.63b 912.80 ± 23.94a 939.49 ± 12.44a 14 3-Methyl-1-butanol dimer C5H12O 1213.3 414.364 1.49166 RI, DT, IS 439.90 ± 29.40c 8572.27 ± 60.56b 9083.14 ± 193.19a 9152.25 ± 137.80a 15 1-Butanol C4H10O 1147.2 348.949 1.18073 RI, DT, IS 157.33 ± 9.44b 198.92 ± 3.92a 152.78 ± 10.85b 156.02 ± 9.80b 16 1-Butanol dimer C4H10O 1146.8 348.54 1.38109 RI, DT, IS 24.14 ± 2.15c 274.75 ± 12.60a 183.02 ± 17.72b 176.80 ± 19.80b 24 1-Propanol C3H8O 1040.9 274.803 1.11042 RI, DT, IS 173.73 ± 4.75a 55.84 ± 2.16c 80.80 ± 4.99b 83.57 ± 2.34b 25 1-Propanol dimer C3H8O 1040.4 274.554 1.24784 RI, DT, IS 58.20 ± 1.30b 541.37 ± 11.94a 541.33 ± 15.57a 538.84 ± 9.74a 28 Ethanol C2H6O 930.6 231.504 1.11901 RI, DT, IS 5337.84 ± 84.16c 11324.05 ± 66.18a 9910.20 ± 100.76b 9936.10 ± 101.24b 34 Methanol CH4O 903.6 223.79 0.98374 RI, DT, IS 662.08 ± 13.87a 76.94 ± 2.15b 61.92 ± 1.96c 62.89 ± 0.81c 37 2-Methyl-1-propanol C4H10O 1098.5 306.889 1.35839 RI, DT, IS 306.91 ± 4.09c 3478.35 ± 25.95a 3308.79 ± 61.75b 3313.85 ± 60.88b 48 1-Pentanol C5H12O 1257.6 470.317 1.25222 RI, DT, IS 26.13 ± 2.52c 116.50 ± 3.71ab 112.37 ± 6.26b 124.17 ± 7.04a Esters 1 Methyl salicylate C8H8O3 1859.6 1616.201 1.20489 RI, DT, IS 615.00 ± 66.68a 485.08 ± 31.30b 470.14 ± 23.02b 429.12 ± 33.74b 7 Butyl hexanoate C10H20O2 1403.0 727.561 1.47354 RI, DT, IS 95.83 ± 17.04a 62.87 ± 3.62a 92.59 ± 11.88b 82.13 ± 3.61c 10 Hexyl acetate C8H16O2 1298.6 524.366 1.40405 RI, DT, IS 44.72 ± 8.21a 33.18 ± 2.17d 41.50 ± 4.38c 40.89 ± 4.33b 11 Propyl hexanoate C9H18O2 1280.9 499.577 1.39274 RI, DT, IS 34.65 ± 3.90d 70.43 ± 5.95a 43.97 ± 4.39b 40.12 ± 4.05c 18 Ethyl hexanoate C8H16O2 1237.4 444.749 1.80014 RI, DT, IS 55.55 ± 5.62c 1606.16 ± 25.63a 787.24 ± 16.95b 788.91 ± 28.50b 20 Isoamyl acetate C7H14O2 1127.8 332.164 1.30514 RI, DT, IS 164.22 ± 1.00d 243.69 ± 8.37c 343.51 ± 13.98b 365.46 ± 1.60a 21 Isoamyl acetate dimer C7H14O2 1126.8 331.345 1.75038 RI, DT, IS 53.61 ± 4.79d 4072.20 ± 11.94a 2416.70 ± 49.84b 2360.46 ± 43.29c 26 Isobutyl acetate C6H12O2 1020.5 263.605 1.23281 RI, DT, IS 101.65 ± 1.81a 15.52 ± 0.67c 44.87 ± 3.21b 45.96 ± 1.41b 27 Isobutyl acetate dimer C6H12O2 1019.6 263.107 1.61607 RI, DT, IS 34.60 ± 1.05d 540.84 ± 5.64a 265.54 ± 8.31c 287.06 ± 3.66b 30 Ethyl acetate dimer C4H8O2 885.2 218.564 1.33587 RI, DT, IS 1020.75 ± 6.86d 5432.71 ± 6.55a 5052.99 ± 9.65b 5084.47 ± 7.30c 31 Ethyl acetate C4H8O2 878.3 216.574 1.09754 RI, DT, IS 215.65 ± 3.58a 38.29 ± 2.37c 71.59 ± 2.99b 69.32 ± 2.85b 32 Ethyl formate C3H6O2 838.1 205.127 1.19738 RI, DT, IS 175.48 ± 3.79d 1603.20 ± 13.72a 1472.10 ± 5.95c 1509.08 ± 13.26b 35 Ethyl octanoate C10H20O2 1467.0 852.127 1.47312 RI, DT, IS 198.86 ± 36.71b 1853.06 ± 17.60a 1555.51 ± 24.21a 1478.05 ± 33.63a 36 Ethyl octanoate dimer C10H20O2 1467.0 852.127 2.03169 RI, DT, IS 135.50 ± 13.02d 503.63 ± 15.86a 342.89 ± 11.62b 297.28 ± 14.40c 38 Ethyl butanoate C6H12O2 1042.1 275.479 1.5664 RI, DT, IS 21.29 ± 2.68c 1384.67 ± 8.97a 1236.52 ± 20.21b 1228.09 ± 5.09b 39 Ethyl 3-methylbutanoate C7H14O2 1066.3 288.754 1.26081 RI, DT, IS 9.70 ± 1.85d 200.29 ± 4.21a 146.87 ± 8.70b 127.13 ± 12.54c 40 Propyl acetate C5H10O2 984.7 246.908 1.48651 RI, DT, IS 4.57 ± 1.07c 128.63 ± 4.28a 87.75 ± 3.26b 88.49 ± 1.99b 41 Ethyl propanoate C5H10O2 962.1 240.47 1.46051 RI, DT, IS 10.11 ± 0.34d 107.08 ± 3.50a 149.60 ± 5.39c 167.15 ± 12.90b 42 Ethyl isobutyrate C6H12O2 971.7 243.229 1.56687 RI, DT, IS 18.29 ± 2.61d 55.22 ± 1.07c 98.81 ± 4.67b 104.71 ± 4.73a 43 Ethyl lactate C5H10O3 1352.2 628.782 1.14736 RI, DT, IS 31.81 ± 2.91c 158.03 ± 2.80b 548.14 ± 74.21a 527.01 ± 39.06a 44 Ethyl lactate dimer C5H10O3 1351.9 628.056 1.53618 RI, DT, IS 44.55 ± 2.03c 47.56 ± 4.02c 412.23 ± 50.96a 185.87 ± 31.25b 47 Ethyl heptanoate C9H18O2 1339.7 604.482 1.40822 RI, DT, IS 39.55 ± 6.37a 38.52 ± 2.47a 28.44 ± 1.52c 30.77 ± 2.79b Unknown 1 RI, DT, IS 15.53 ± 0.18 35.69 ± 0.80 12.70 ± 0.80 10.57 ± 0.86 2 RI, DT, IS 36.71 ± 1.51 120.41 ± 3.44 198.12 ± 6.01 201.19 ± 3.70 3 RI, DT, IS 44.35 ± 0.88 514.12 ± 4.28 224.78 ± 6.56 228.32 ± 4.62 4 RI, DT, IS 857.64 ± 8.63 33.22 ± 1.99 35.05 ± 5.99 35.17 ± 3.97 * Represents the retention index calculated using n-ketones C4−C9 as external standard on MAX-WAX column. ** Represents the retention time in the capillary GC column. *** Represents the migration time in the drift tube. Analysis of the change in volatile organic compounds (VOCs) during fermentation by GS-IMS
-
This study adopted the GC-IMS method to test the volatile organic compounds (VOCs) in the samples from the different fermentation stages of Marselan wine. Figure 1 shows the gas phase ion migration spectrum obtained, in which the ordinate represents the retention time of the gas chromatographic peaks and the abscissa represents the ion migration time (normalized)[16]. The entire spectrum represents the aroma fingerprints of Marselan wine at different fermentation stages, with each signal point on the right of the relative reactant ion peak (RIP) representing a volatile organic compound detected from the sample[17]. Here, the sample in stage 1 (juice processing) was used as a reference and the characteristic peaks in the spectrum of samples in other fermentation stages were compared and analyzed after deducting the reference. The colors of the same component with the same concentration cancel each other to form a white background. In the topographic map of other fermentation stages, darker indicates higher concentration compared to the white background. In the 2D spectra of different fermentation stages, the position and number of peaks indicated that peak intensities are basically the same, and there is no obvious difference. However, it is known that fermentation is an extremely complex chemical process, and the content and types of volatile organic compounds change with the extension of fermentation time, so other detection and characterization methods are needed to make the distinction.
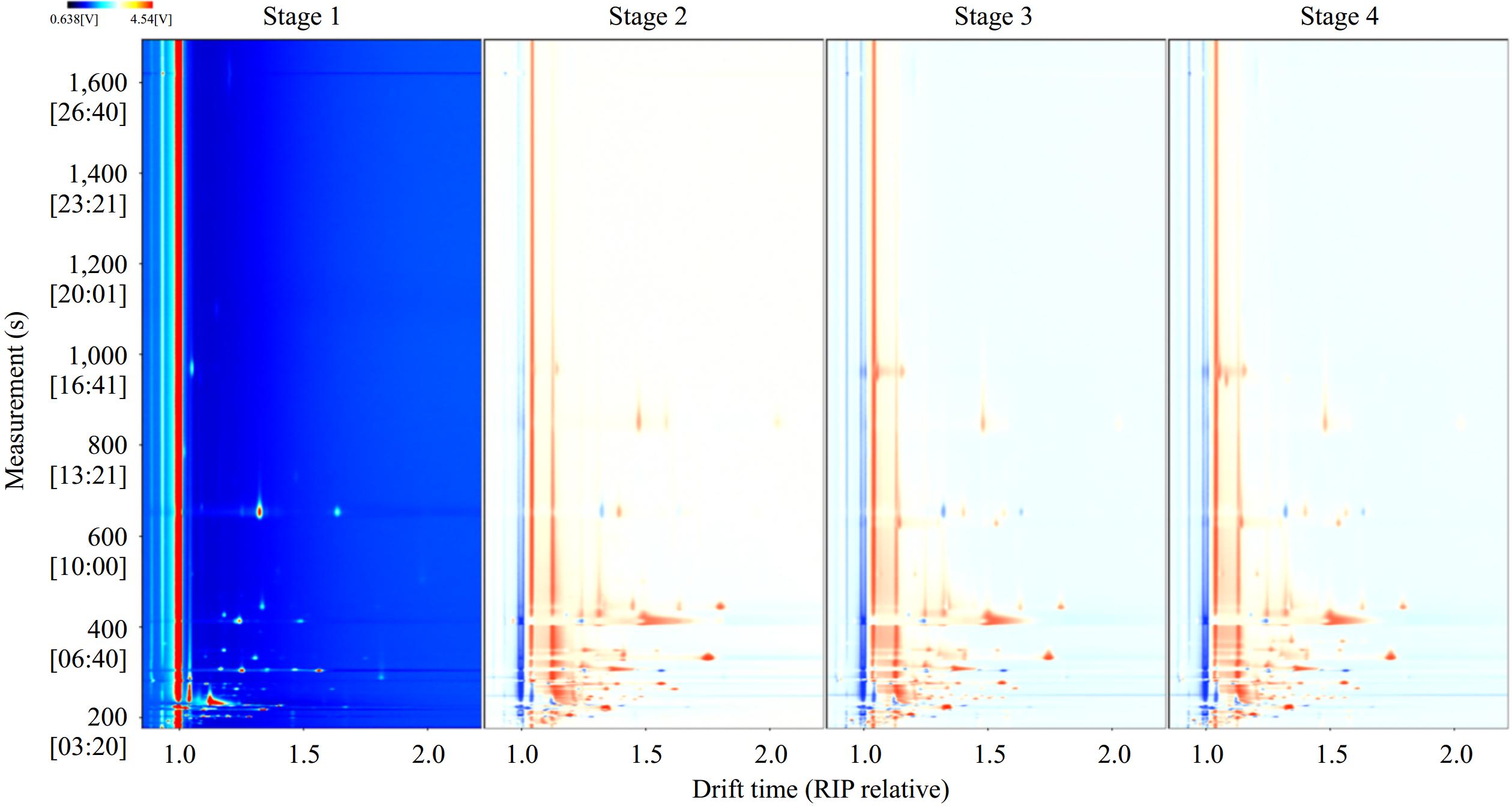
Figure 1.
2D-topographic plots of volatile organic compounds in Marselan wine at different fermentation stages.
Fingerprint analysis of VOCs at different fermentation stages of Marselan wine
-
To visually display the dynamic changes of various substances in the fermentation process of Marselan wine, peaks with obvious differences were extracted to form the characteristic fingerprints for comparison (Fig. 2). Each row represents all signal peaks selected from samples at the same stage, and each column means the signal peaks of the same volatile compound in samples from different fermentation stages. Figure 2 shows the volatile organic compounds (VOCs) information for each sample and the differences between samples, where the numbers represent the undetermined substances in the migration spectrum library. The changes of volatile substances in the process of Marselan winemaking is observed by the fingerprint. As shown in Fig. 2 and Table 2, a total of 40 volatile chemical components were detected by qualitative analysis according to their retention time and ion migration time in the HS-GC-IMS spectrum, including 17 esters, eight alcohols, eight aldehydes, two ketones, one organic acid, and four unanalyzed flavor substances. The 12 volatile organic compounds presented dimer due to ionization of the protonated neutral components before entering the drift tube[18]. As can be seen from Table 2, the VOCs in the winemaking process of Marselan wine are mainly composed of esters, alcohols, and aldehydes, which play an important role in the construction of aroma characteristics.
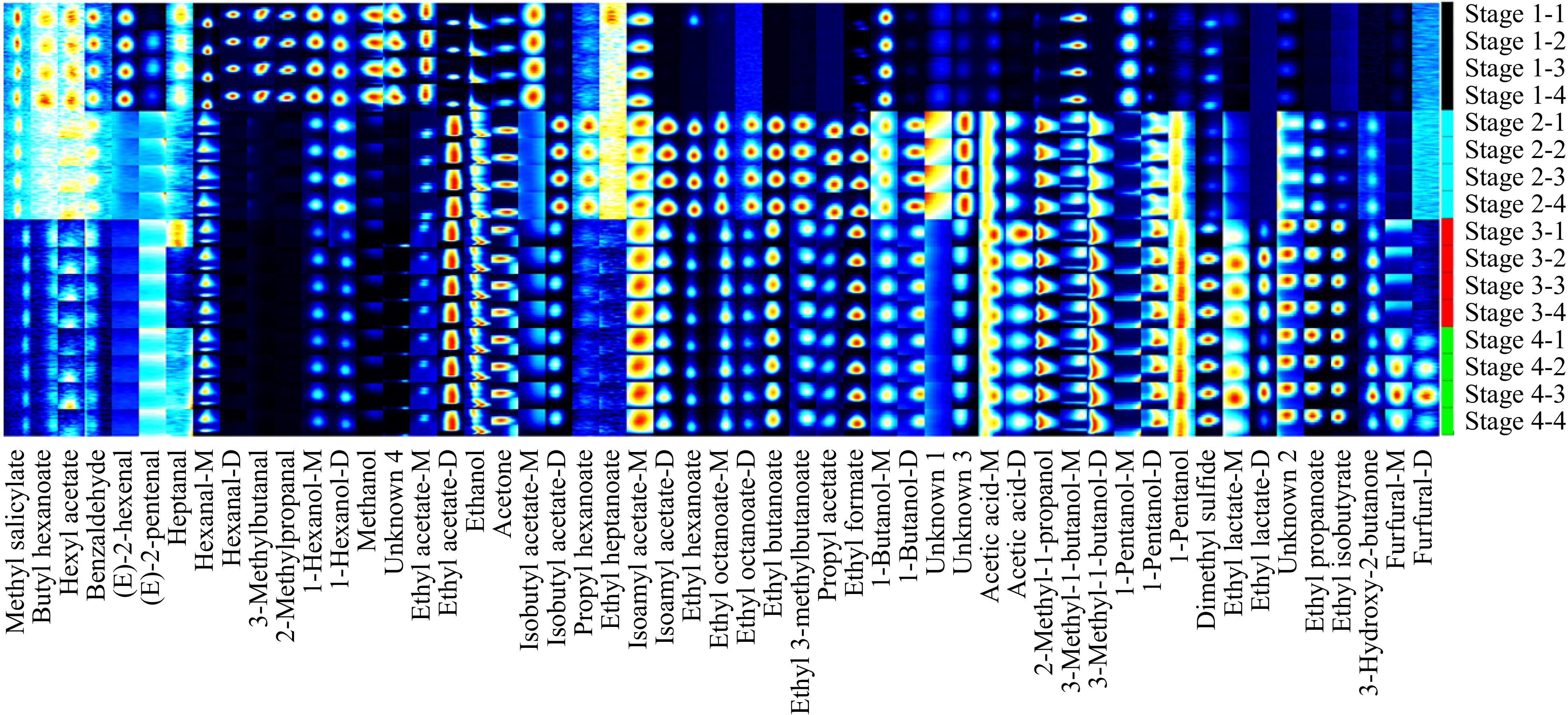
Figure 2.
Fingerprints of volatile organic compounds in Marselan wine at different fermentation stages.
Table 2. Antioxidant activity, total polyphenols, and flavonoids content of Marselan wine at different fermentation stages.
Winemaking stage TFC (mg CE/L) TPC (mg GAE/L) FRAP (mM FeSO4/mL) ABTs (mM Trolox/L) Stage 1 315.71 ± 0.00d 1,083.93 ± 7.79d 34.82c 38.92 ± 2.12c Stage 2 1,490.00 ± 7.51c 3,225.51 ± 53.27c 77.32b 52.17 ± 0.95b Stage 3 1,510.00 ± 8.88a 3,307.143 ± 41.76b 77.56b 53.04 ± 0.76b Stage 4 1,498.57 ± 6.34b 3,370.92 ± 38.29a 85.07a 57.46 ± 2.55a Means in the same column with different letters are significantly different (p < 0.05). Esters are produced by the reaction of acids and alcohols in wine, mainly due to the activity of yeast during fermentation[19], and are the main components of fruit juices and wines that produce fruit flavors[20,21]. In this study, it was found that they were the largest detected volatile compound group in Marselan wine samples, which is consistent with previous reports[22]. It can be observed from Table 2 that the contents of most esters increased gradually with the extension of fermentation time, and they mainly began to accumulate in large quantities during the stage of alcohol fermentation. The contents of ethyl hexanoate (fruity), isoamyl acetate (banana, pear), ethyl octanoate (fruity, pineapple, apple, brandy), ethyl acetate (fruity), ethyl formate (spicy, pineapple), and ethyl butanoate (sweet, pineapple, banana, apple) significantly increased at the stage of alcoholic fermentation and maintained a high level in the subsequent fermentation stage (accounting for 86% of the total detected esters). These esters can endow a typical fruity aroma of Marselan wine, and played a positive role in the aroma profiles of Marselan wine. Among them, the content of ethyl acetate is the highest, which is 5,153.79 μg/mL in the final fermentation stage, accounting for 33.6% of the total ester. However, the content of ethyl acetate was relatively high before fermentation, which may be from the metabolic activity of autochthonous microorganisms present in the raw materials. Isobutyl acetate, ethyl 3-methyl butanoate, propyl acetate, ethyl propanoate, ethyl isobutyrate, and ethyl lactate were identified and quantified in all fermentation samples. The total contents of these esters in stage 1 and 4 were 255.28 and 1,533.38 μg/mL, respectively, indicating that they may also have a potential effect on the aroma quality of Marselan wine. The results indicate that esters are an important factor in the formation of flavor during the brewing process of Marselan wine.
Alcohols were the second important aromatic compound in Marselan wine, which were mainly synthesized by glucose and amino acid decomposition during alcoholic fermentation[23,24]. According to Table 2, eight alcohols including methanol, ethanol, propanol, butanol, hexanol, amyl alcohol, 3-methyl-1-butanol, and 2-methyl-1-propanol were detected in the four brewing stages of Marselan wine. The contents of ethanol (slightly sweet), 3-methyl-1-butanol (apple, brandy, spicy), and 2-methyl-1-propanol (whiskey) increased gradually during the fermentation process. The sum of these alcohols account for 91%−92% of the total alcohol content, which is the highest content of three alcohols in Marselan wine, and may be contributing to the aromatic and clean-tasting wines. On the contrary, the contents of 1-hexanol and methanol decreased gradually in the process of fermentation. Notably, the content of these rapidly decreased at the stage of alcoholic fermentation, from 2,026.07 to 1,218.98 μg/mL and 662.08 to 76.94 μg/mL, respectively, which may be ascribed to volatiles changed from alcohols to esters throughout fermentation. The reduction of the concentration of some alcohols also alleviates the strong odor during wine fermentation, which plays an important role in the improvement of aroma characteristics.
Acids are mainly produced by yeast and lactic acid bacteria metabolism at the fermentation stage and are considered to be an important part of the aroma of wine[22]. Only one type of acid (acetic acid) was detected in this experiment, which was less than previously reported, which may be related to different brewing processes. Acetic acid content is an important factor in the balance of aroma and taste of wine. Low contents of volatile acids can provide a mild acidic smell in wine, which is widely considered to be ideal for producing high-quality wines. However, levels above 700 μg/mL can produce a pungent odor and weaken the wine's distinctive flavor[25]. The content of acetic acid increased first and then decreased during the whole fermentation process. The content of acetic acid increased rapidly in the second stage, from 719.91 to 3,914.55 μg/mL reached a peak in the third stage (5,161.81 μg/mL), and decreased to 4,630.65 μg/mL in the last stage of fermentation. Excessive acetic acid in Marselan wine may have a negative impact on its aroma quality.
It was also found that the composition and content of aldehydes produced mainly through the catabolism of amino acids or decarboxylation of ketoacid were constantly changing during the fermentation of Marselan wines. Eight aldehydes, including furfural, hexanal, heptanal, 2-methylpropanal, 3-methylbutanal, dimethyl sulfide, (E)-2-hexenal, and (E)-2-pentenal were identified in all stage samples. Among them, furfural (caramel bread flavor) and hexanal (grass flavor) are the main aldehydes in Marselan wine, and the content increases slightly with the winemaking process. While other aldehydes such as (E)-2-hexenal (green and fruity), 3-methylbutanol (fresh and malt), and 2-methylpropanal (fresh and malt) were decomposed during brewing, reducing the total content from 536.52 to 85.15 μg/mL, which might potently affect the final flavor of the wine. Only two ketones, acetone, and 3-hydroxy-2-butanone, were detected in the wine samples, and their contents had no significant difference in the fermentation process, which might not affect the flavor of the wine.
Multivariate statistical analysis
-
To more intuitively analyze the differences of volatile organic compounds in different brewing stages of Marselan wine samples, principal component analysis was performed[26−28]. As presented in Fig. 3, the points corresponding to one sample group were clustered closely on the score plot, while samples at different fermentation stages were well separated in the plot. PC1 (79%) and PC2 (18%) together explain 97% of the total variance between Marselan wine samples, indicating significant changes in volatile compounds during the brewing process. As can be seen from the results in Fig. 3, samples of stages 1, 2, and 3 can be distinguished directly by PCA, suggesting that there are significant differences in aroma components in these three fermentation stages. Nevertheless, the separation of stage 3 and stage 4 samples is not very obvious and both presented in the same quadrant, which means that their volatile characteristics were highly similar, indicating that the volatile components of Marselan wine are formed in stage 3 during fermentation (Fig. S1). The above results prove that the unique aroma fingerprints of the samples from the distinct brewing stages of Marselan wine were successfully constructed using the HS-GC-IMS method.
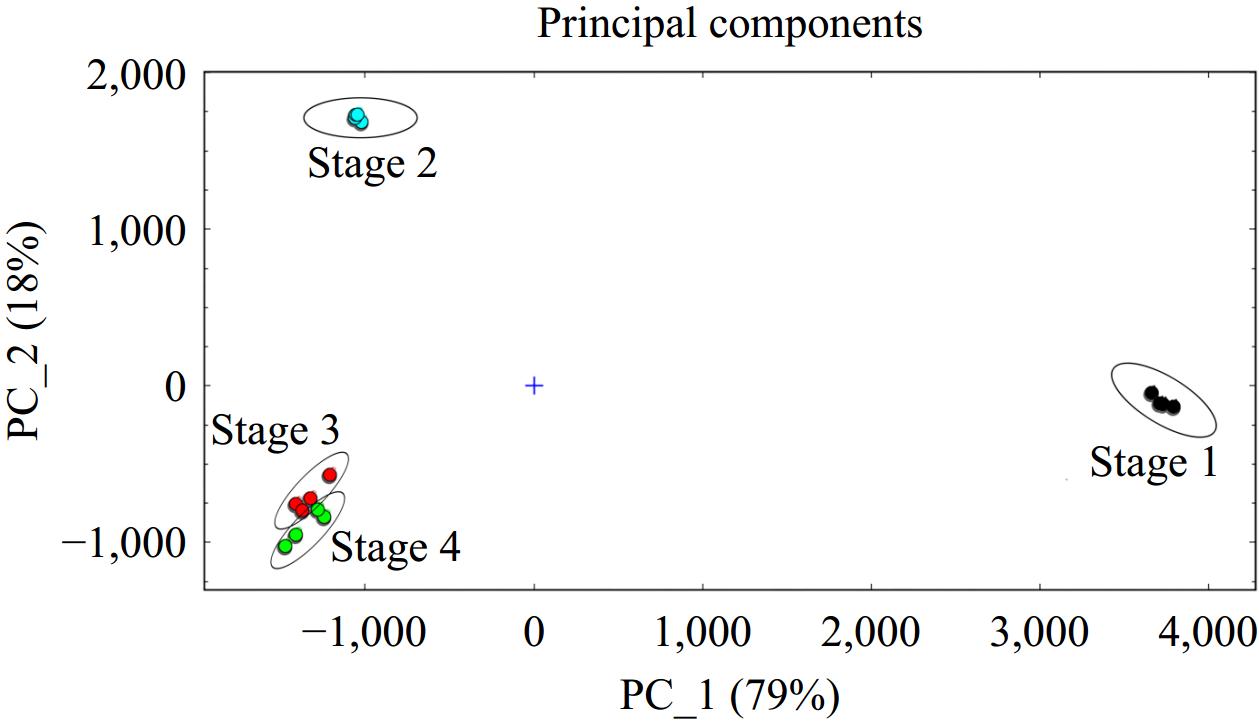
Figure 3.
PCA based on the signal intensity obtained with different fermentation stages of Marselan wine.
Based on the results of the PCA, OPLS-DA was used to eliminate the influence of uncontrollable variables on the data through permutation test, and to quantify the differences between samples caused by characteristic flavors[28]. Figure 4 revealed that the point of flavor substances were colored according to their density and the samples obtained at different fermentation stages of wine have obvious regional characteristics and good spatial distribution. In addition, the reliability of the OPLS-DA model was verified by the permutation method of 'Y-scrambling'' validation. In this method, the values of the Y variable were randomly arranged 200 times to re-establish and analyze the OPLS-DA model. In general, the values of R2 (y) and Q2 were analyzed to assess the predictability and applicability of the model. The results of the reconstructed model illustrate that the slopes of R2 and Q2 regression lines were both greater than 0, and the intercept of the Q2 regression line was −0.535 which is less than 0 (Fig. 5). These results indicate that the OPLS-DA model is reliable and there is no fitting phenomenon, and this model can be used to distinguish the four brewing stages of Marselan wine.
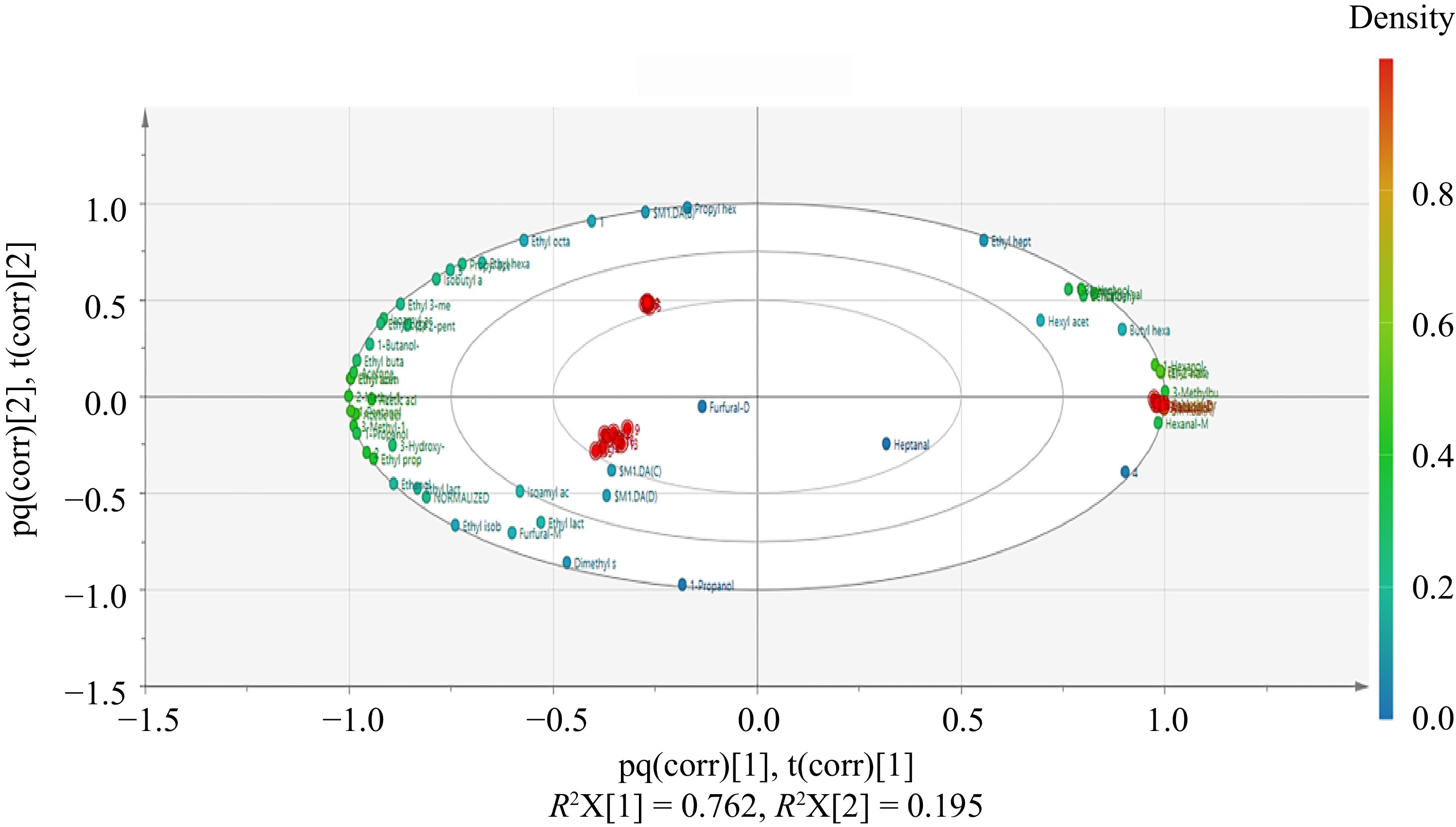
Figure 4.
Scores plot of OPLS-DA model of volatile components in Marselan wine at different fermentation stages.
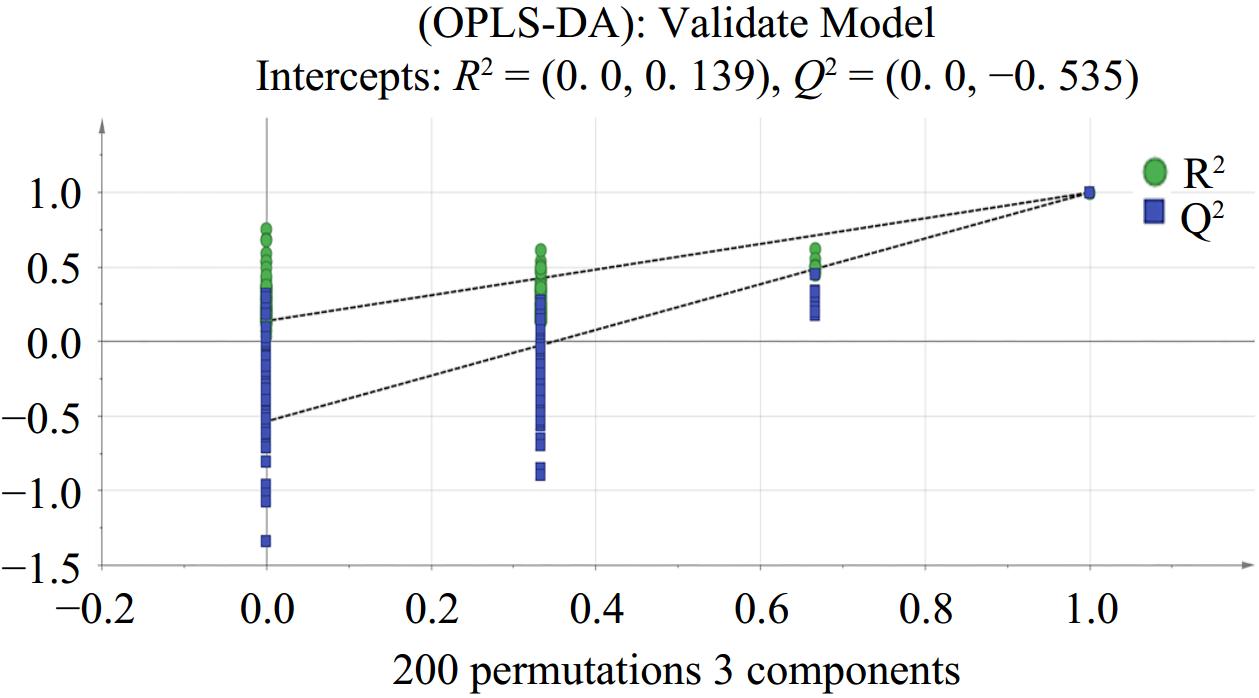
Figure 5.
Permutation test of OPLS-DA model of volatile components in Marselan wine at different fermentation stages (n = 200).
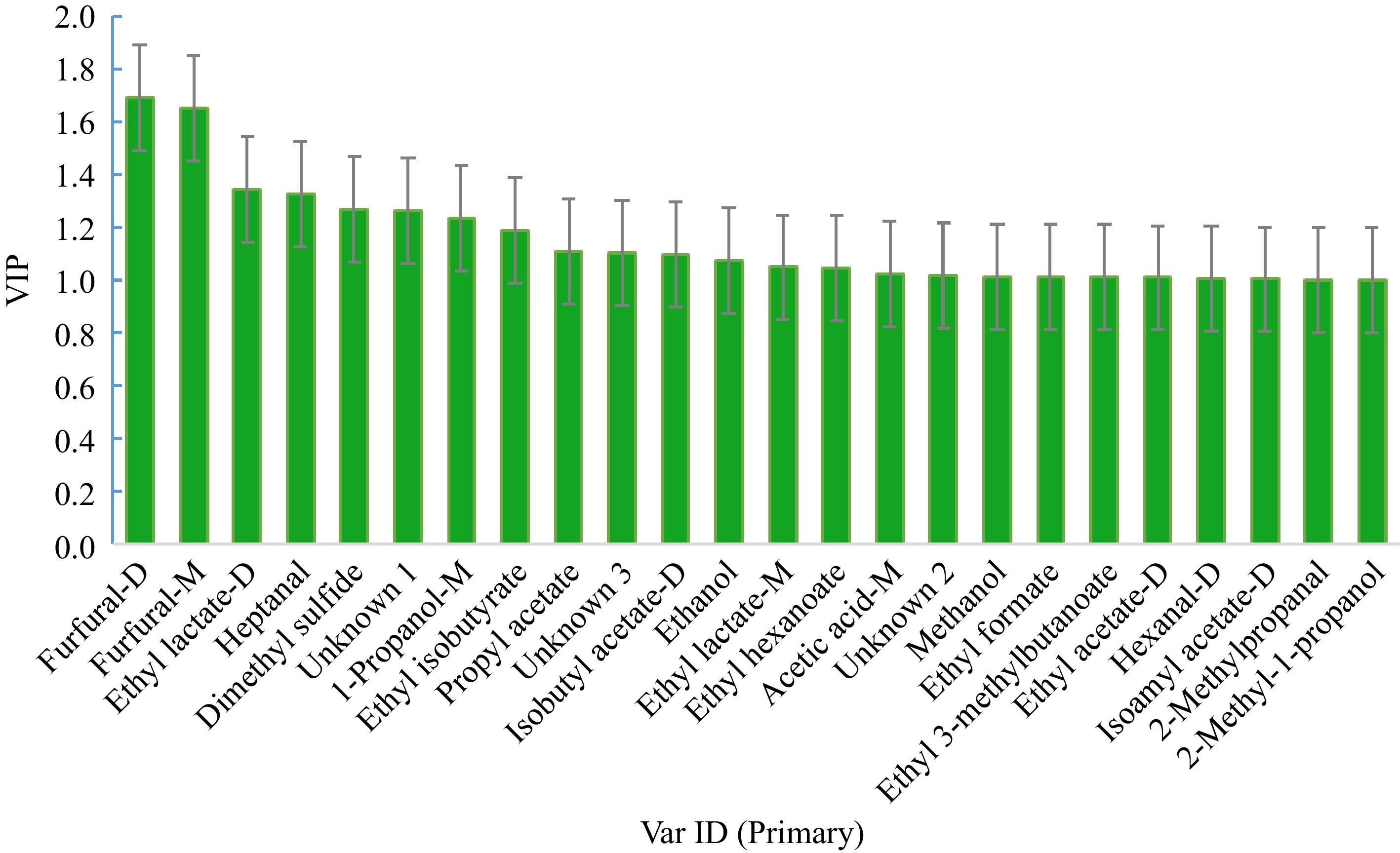
VIP is the weight value of OPLS-DA model variables, which was used to measure the influence intensity and explanatory ability of accumulation difference of each component on classification and discrimination of each group of samples. In previous studies, VIP > 1 is usually used as a screening criterion for differential volatile substances[28−30]. In this study, a total of 22 volatile substances had VIP values above 1, indicating that these volatiles could function as indicators of Marselan wine maturity during fermentation (see Fig. 6). These volatile compounds included furfural, ethyl lactate, heptanal, dimethyl sulfide, 1-propanol, ethyl isobutyrate, propyl acetate, isobutyl acetate, ethanol, ethyl hexanoate, acetic acid, methanol, ethyl formate, ethyl 3-methylbutanoate, ethyl acetate, hexanal, isoamyl acetate, 2-methylpropanal, 2-methyl-1-propanol, and three unknown compounds.
-
This study focuses on the change of volatile flavor compounds and antioxidant activity in Marselan wine during different brewing stages. A total of 40 volatile aroma compounds were identified and collected at different stages of Marselan winemaking. The contents of volatile aroma substances varied greatly at different stages, among which alcohols and esters were the main odors in the fermentation stage. The proportion of furfural was small, but it has a big influence on the wine flavor, which can be used as one of the standards to measure wine flavor. Flavonoids and phenols were not only factors of flavor formation, but also important factors to improve the antioxidant capacity of Marselan wine. In this study, the aroma of Marselan wines in different fermentation stages was analyzed, and its unique aroma fingerprint was established, which can provide accurate and scientific judgment for the control of the fermentation process endpoint, and has certain guiding significance for improving the quality of Marselan wines (Table S1). In addition, this work will provide a new approach for the production management of Ningxia's special wine as well as the development of the native Chinese wine industry.
This work were supported by the project of Hainan Province Science and Technology Special Fund (ZDYF2023XDNY031) and the Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences in China (Grant No. 1630122022003).
-
The authors confirm contribution to the paper as follows: study conception and design: Gong X, Fang L; data collection: Fang L, Li Y; analysis and interpretation of results: Qi N, Chen T; draft manuscript preparation: Fang L. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Fang L, Qi N, LI Y, Chen T, Gong X. 2024. Changes in the physicochemical and volatile profiles during the winemaking of Marselan in the Eastern Foot of Helan Mountain, China. Food Innovation and Advances 3(4): 396−404 doi: 10.48130/fia-0024-0039
Changes in the physicochemical and volatile profiles during the winemaking of Marselan in the Eastern Foot of Helan Mountain, China
- Received: 29 August 2024
- Revised: 07 November 2024
- Accepted: 25 November 2024
- Published online: 11 December 2024
Abstract: Marselan wine, one of the most important wines in the Ningxia Hui Autonomous Region of China, has attracted much attention due to its unique quality. This study focused on determining and analyzing the changes in volatile flavor compounds and antioxidant activity during different stages of Marselan winemaking. A total of 40 volatile aroma compounds were identified by headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). Among these compounds, ethyl hexanoate, isoamyl acetate, ethyl formate, ethyl acetate, ethyl butanoate, ethyl octanoate, 3-methyl-1-butanol, ethanol, and 2-methyl-1-propanol showed significant increases after fermentation. Flavonoid and phenol contents in Marselan wine samples also significantly increased after fermentation, demonstrating high antioxidant capacity. Principal component analysis (PCA) successfully distinguished the fruit juice processing stage, alcohol fermentation stage, and malolactic fermentation stage, while the malolactic fermentation stage and wine stable stage could not be distinguished, This indicates that the formation of aroma profiles primarily occurs during the malolactic fermentation stage. The study successfully established flavor fingerprints of samples from different stages of Marselan wine production based on the detected volatile compounds.