-
Naturally sourced bioactive compounds have gained considerable attention in the nutraceutical industry due to consumers' preference for natural ingredients. There has been a global trend toward the usage of natural substances in medical and dietary plants as sources of bioactive compounds due to rising risk factors for humans to contract numerous lethal diseases such as cancer and cardiovascular diseases, metabolic disorders, and neurodegenerative disorders[1]. The use of naturally derived compounds in the food and therapeutic industries would be a promising alternative to their synthetic counterparts in terms of low cost, high compatibility with dietary intake, and low to no harmful effects on the human body[2].
Sugarcane (Saccharum officinarum L.) processing is one of the major industries in the Philippines, contributing to the country's economy of no less than PhP 70 billion[3]. Desugared sugarcane extract (DSE) is one of the by-products of the sugar refining process and is obtained from a series of adsorption-desorption processes in ionic exchange resins via a CTi patented process[4]. Many studies have shown that these extracts contain high levels of oxygen radical absorbance capacity (ORAC) values of 1,000 units per gram owing to the phytochemical compounds present, such as polyphenolics, including flavonoids, benzenoids, lignans, and neolignans, exhibiting antioxidant, anti-inflammatory, anti-atherosclerotic, and tyrosinase-inhibiting properties[5−7].
Apart from the diverse functionality of the sugarcane extracts, the beneficial effects of these compounds still depend on their bioaccessibility and bioavailability in the digestive tract, which is a critical factor to be considered to render its physiological function[8]. Bioaccessibility indicates the effect of gastrointestinal digestion on compounds from the food that become accessible for absorption. Phenolic compounds are easily degradable in the gastrointestinal environment, where they can be metabolized into compounds with different biological properties (enzymatic action, pH, and gut microbiota), resulting in lower bioaccessibility[9]. This was observed in the study on phenolic compounds from sweet orange, wherein the bioaccessibility of intestinal phenolic compounds decreased by 25%[10]. Therefore, in vitro gastrointestinal digestion assays are essential to simulate the physiological conditions of the human body and their effect on the bioaccessibility of the phytochemicals under study.
Hence, in support of the extensive functionality assessment of the extract from sugarcane, this work aims to assess other nutraceutical prospects of desugared sugarcane extract, such as phenolic acid bioaccessibility and bioactivity under extended simulated gastrointestinal digestion, antioxidant, antibacterial activities against foodborne pathogens, and antidiabetic functionality via in vitro assays.
-
Desugared sugarcane extracts (DSE) were obtained from Forever Nutriliving Corp., Sagay City, Negros Occidental, Philippines, through a patented process[4]. The process is based on a two-column system that has been tested for the recovery and purification of antioxidant compounds from sugarcane syrup. The obtained DSE was batch validated for purity suitable for food-grade applications by the manufacturer in compliance with Philippine FDA regulations as a Food Manufacturer. All standards and reagents used were obtained commercially. Bacterial strains used in the antibacterial assay (S. aureus, B. cereus, E. coli, Salmonella sp., and P. aeruginosa) were obtained from the Philippine National Collection of Microorganisms (PNCM), BIOTECH, UPLB, Laguna, Philippines.
Proximate composition of DSE
-
Proximate composition (moisture, ash, crude protein, crude fat, and crude fiber) of DSE samples were analyzed following the standard method of AOAC[11]. Moisture and ash content were analyzed using a convection-type oven (Memmert UM100, Island, Skandinavia) and muffle furnace (Thermolyne F6010, Thermo Scientific, USA), respectively. Crude protein, crude fiber, and crude fat were analyzed using FOSS KjeltecTM 8100, FOSS FT 122 FibertecTM and FOSS SoxtecTM 243 (FOSS Analytical Co., Ltd. Denmark), respectively.
Phytochemical profiling of DSE
-
Phytochemical profiling in terms of total phenolic content (TPC), total flavonoid content (TFC), total anthocyanin content (TAC), and condensed tannins (CT) were evaluated using standard methods. The TPC of DSE samples was analyzed using the Folin-Ciocalteu method as outlined by Singleton et al.[12] and was expressed as gallic acid equivalent (g GAE/100 g sample). Total flavonoid content was analyzed using the AlCl3 method and expressed as quercetin equivalent (g QE/100 g sample)[13]. The TAC and CT were determined via the pH differential method and the vanillin condensation method in an acidic medium following the methods of Shibata et al.[14] and Shay et al.[15], respectively. The TAC was expressed as cyanidin-3-glucoside equivalent (g CYE/100 g sample) whereas CT was expressed as catechin equivalent (g CAT/100 g sample).
Quantitative analysis of the phenolic compounds present in DSE by HPLC
-
Quantitative phenolic acid profiling of DSE samples was conducted using Shimadzu Prominence-i LC-2030C Plus (Shimadzu Corp., Kyoto, Japan) equipped with an Athena C18 column (10 μm, 4.6 mm × 250 mm, ANPEL Laboratory Technologies (Shanghai) Inc., Shanghai, China) following the optimized method of the Oils and Fats Laboratory, BIOTECH, UPLB. A gradient elution program was used using 1% acetic acid (mobile phase A) and acetonitrile (mobile phase B) for p-hydroxybenzoic acid, gallic acid, caffeic acid, p-coumaric acid, sinapic acid, chlorogenic acid, vanillic acid, ferulic acid, and rosmarinic acid. The detection of compounds was set at 272 nm for the rest of the compounds. Analysis was done at 45 ˚C column temperature, flow rate of 1.0 mL/min, and 20 μL injection volume. Quantification was done using external standard calibration from 2 to 10 μg/mL.
Antioxidant activity: DPPH and ABTS radical scavenging activity
-
The capacities of DSE to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-(3-ethylbenzthiazolin-6-sulfonic acid) (ABTS) radicals were assessed following the method of Abbas et al.[16] with slight modifications. Radical solutions of DPPH (0.1 mmol/L in 95% methanol) and ABTS (1.8 mmol/L in 95% methanol) were prepared and allowed to react to DSE at 0−4 mg/mL (in 95% methanol) at room temperature for 30 min. Methanol was used as a blank and ascorbic acid was used as a positive control for the assay. The absorbance of the resulting solution was measured at 516 nm for DPPH and 734 nm for ABTS. The percentage of radical scavenging activity was calculated using Eqn (1):
$ Radical\; scavenging\; activity\; \text{(%)}=\left[1-\left(\dfrac{A_{sample}}{A_{blank}}\right)\right]\times100 $ (1) where, Asample and Ablank are the absorbances of the sample or blank. The dose-dependent graphs of the DSE sample and ascorbic acid (control) were generated, and the EC50 values indicating the concentration at which the sample effectively scavenged 50% of the radicals under the assayed conditions were determined using GraphPad Prism 6 software.
Antioxidant activity: ferric reducing capacity
-
The ferric reducing capacity (FRC) of DSE was evaluated using the phenanthroline method[17]. An aliquot of DSE samples (0−4 mg/mL in 95% methanol) was allowed to react in a 0.2% FeCl3 solution containing 0.5% 1,10-phenanthroline. The resulting solution was incubated at room temperature in the dark for 20 min and the absorbance was measured at 510 nm against a reagent blank. Methanol and ascorbic acid were used as blank and positive controls, respectively. The amount of Fe2+ generated was quantified using a FeSO4 standard curve, and ferric reducing capacity was calculated using Eqn (2):
$ Ferric\;reducing\;capacity\; ({\text{%}})=\left[1-\left(\dfrac{\mu mol\;{Fe}^{2+}}{\mu mol\;{Fe}^{3+}}\right)\right] \times 100 $ (2) where, μmol Fe2+ is the amount of generated ferrous ions quantified using the standard curve; and μmol Fe2+ is the amount of ferric ions initially present in the assay (which is equal to 12.33 μmol under the above assayed conditions). The dose-dependent graphs of the DSE sample and ascorbic acid (control) were generated, and the EC50 values indicating the concentration at which the sample effectively reduced 50% of the ferric ions to ferrous ions under the assayed conditions were determined using GraphPad Prism 6 software.
Bioaccessibility under extended simulated gastrointestinal digestion and dialysability
-
A simulated gastrointestinal digestion, including dialysability, was used to evaluate the bioaccessibility of the dialysable fraction of phenolic compounds in DSE. Dialysability was employed to determine the free soluble phenolic compounds potentially available for further uptake, mimicking cellular uptake through diffusion[18].
For gastric digestion, a 220 mg DSE sample was immersed in a gastric solution containing 50.0 mL 0.90% NaCl, 8.0 mL 0.10 M HCl, and 4.0 mL 40 mg/mL pepsin in 0.10 M HCl, then incubated at 37 °C for 1 h in an incubator shaker (TOU-50, MRC Laboratory Instruments, Harlow, UK). Aliquots of gastric phase were stored at −20 °C until further analysis. Rinsed dialysis tubing (2.54 cm × 10 cm diameter × length, 14 kDa MWCO) was filled with 5.50 mL 0.90% NaCl and 5.50 mL 0.50 mol/L NaHCO3 then suspended in the gastric digesta for 45 min at 37 °C in an incubator shaker, allowing neutralization of pH in gastric digestion. This simulates the transition from the gastric phase to the intestinal phase. The intestinal phase proceeds after adjustment of the pH to 6.5 using 1 mol/L HCl and the addition of 18.0 mL 2 mg/mL pancreatin and 12 mg/mL bile salts in 0.10 mol/L NaHCO3. After 2 h of incubation at 37 °C, aliquots of gastrointestinal fraction (outside the dialysis bag) and dialysed fraction (content of the dialysis bag) were stored at −20 °C until further analysis.
One mg/mL of DSE in 80% methanol was prepared for the determination of undigested phenolics. Sample aliquots were drawn at each step of the digestion process, and the total phenolic content was analyzed[12]. The Bioaccessibility Index of fractions of phenolic compounds in DSE after the digestion phases were calculated using Eqn (3):
$ Bioaccessibility\;Index\; ({\text{%}})=\dfrac{{TPC}_{gastric/intestinal/freesoluble}}{{TPC}_{undigested}} \times 100 $ (3) where, TPCgastric/intestinal/free soluble is the total phenolic content of the aliquot obtained after gastric, intestinal digestion, and dialysable fraction; TPCundigested is the total phenolic content of the aliquot of the undigested sample.
Antibacterial activity
-
The antibacterial inhibitory activity of DSE samples against common food borne disease causing bacteria such as S. aureus, B. cereus, E. coli, Salmonella sp., and P. aeruginosa were qualitatively measured using the disc diffusion method in Mueller Hinton Agar (MHA) plates[19]. The test organisms were inoculated in nutrient broth and incubated overnight at 37 °C. Inoculums were diluted in Meuller Hinton Broth and standardized to 0.5 McFarland Standard corresponding to 1.5 × 108 CFU/mL. Diluted inoculums were uniformly spread onto individual MHA plates using sterile cotton swabs. Sterilized filter paper discs of 6 mm in diameter were dipped in each of 0.1, 1.0 and 10.0 mg/mL of DSE (in 95% methanol) and placed on agar medium in Petri plates at their labeled positions. Chloramphenicol and 95% methanol were used as positive and diluent controls, respectively. Zones of inhibition were measured after overnight incubation of plates at 37 °C.
Alpha-glucosidase inhibition activity
-
The α-glucosidase inhibitory activity of sugarcane extract was carried out according to the standard method with minor modifications[20]. The inhibition of the α-glucosidase enzyme by DSE (0.1−0.5 mg/mL in distilled water) was determined in 100 mmol/L sodium phosphate buffer pH 6.8 by reacting 1 U/mL of the enzyme with 1.25 mmol/L 4-Nitrophenyl-β-D-glucopyranoside at 37 °C. The reaction was terminated after 10 min by adding 0.1 M Na2CO3. The absorbance of the released p-nitrophenol was measured at 405 nm. Distilled water and ascorbic acid were used as blank and positive controls, respectively. The inhibition activity was calculated using Eqn (4):
$ \alpha {\text-}glucosidase\;inhibition\;activity\;{\text{(%)}}=\left[1-\left(\dfrac{{A}_{sample}}{{A}_{blank}}\right)\right] \times 100 $ (4) where, Asample and Ablank are the absorbances of the sample and blank, respectively. The dose-dependent graphs of the DSE sample and ascorbic acid (control) were generated and the IC50 values indicating the concentration at which the sample inhibits 50% of the α-glucosidase activity under the assayed conditions were determined using GraphPad Prism 6 software.
Statistical analysis
-
Data were expressed as the mean values of at least three replicates and standard deviations were statistically analyzed by performing a one-way ANOVA. Differences among group means were compared using Tukey's post hoc comparison test using R software (version 4.2.1, R Foundation for Statistical Computing, Vienna, Austria). Significant differences between means were determined at p < 0.05. The EC50 or IC50 value determination from the dose-dependent graphs were calculated using GraphPad Prism 6 software (Boston, MA 02110, USA).
-
Table 1 presents the proximate composition, phytochemical compounds, and phenolic profiles of DSE, a concentrated extract derived from sugarcane that has undergone a process to remove sugars, leaving behind other bioactive compounds. The extract contains appreciable amounts of protein, with 5.98% suggesting the possible co-extraction of proteins during the refining process. This protein content indicates that DSE could serve as a supplementary protein source, potentially beneficial in diets that require additional protein intake. The ash content of DSE is approximately 3.28%, indicating a significant presence of minerals and trace elements. These minerals and trace elements are essential for various biochemical processes and overall health maintenance. Additionally, DSE exhibits relatively low crude fiber and fat content, ranging from 0.06% to 0.07%. These values align closely with the findings of Saska & Chou[6], who reported crude fiber and fat content of around 0.14%−0.19%. The low fiber and fat contents suggest that while DSE is not a significant source of dietary fiber or fat, other nutritional and bioactive components, such as proteins and minerals, enhance its potential as a functional food ingredient.
Table 1. Proximate composition, phytochemical profiling, and phenolic acid quantification in desugared sugarcane extract.
Composition Parameters Moisture 2.50% ± 0.69% Ash 3.28% ± 0.01% Crude protein 5.98% ± 0.03% Crude fiber 0.06% ± 0.00 Crude fat 0.07% ± 0.02% Phytochemical compounds Total phenolic content (g GAE/100 g) 32.21 ± 0.59 Total flavonoid content (mg QE/100 g) 4.50 ± 0.2 Total anthocyanin content (mg CYE/ 100 g) ND Condensed tannins content (mg CE/ 100 g) ND Phenolic acid profiling (mg/100 mg sample)a p-Hydroxybenzoic acid 172.15 ± 1.70 Gallic acid 101.48 ± 2.47 p-Coumaric acid 390.65 ± 5.00 Sinapic acid 638.37 ± 19.32 Ferulic acid ND Rosmarinic acid ND Caffeic acid ND Chlorogenic acid ND Vanillic acid 724.56 ± 15.25 ND – not detected; a HPLC quantification (Linearity and Pearson correlation coefficient of standards). p-hydroxybenzoic acid: y = 74,436.78x – 43,860.17, R2 = 0.9972; Gallic acid: y = 32,740.20x – 15,807.20, R2 = 0.9980; p-coumaric acid: y = 75,863.84x – 28,155.51, R2 = 0.9940; Sinapic acid: y = 15,554.05x + 11,652.20, R2 = 0.9917; Ferulic acid: y= 38,241.65x – 26421.30, R2 = 0.9915; Rosmarinic acid: y = 26,606.15x + 3,964.90, R2 = 0.9815; Caffeic acid: y = 44,829.65x + 22,565.10, R2 = 0.9886; Chlorogenic acid: y = 15,347.45x – 12,230.50, R2 = 0.9975; Vanillic acid: y = 47,194.62x + 928.95, R2 = 0.9955. Phytochemical screening of DSE reveals a rich profile of phenolic compounds, as shown in Table 1. The extract contains a total phenolic content of 32.21 g GAE/100 g, and a flavonoid content of 4.50 mg QE/100 g, while anthocyanins and condensed tannins were not detected. Profiling and quantification of the phenolic acids present in DSE using HPLC identified vanillic, sinapic, and p-coumaric acids as the major phenolic compounds, with 724.56, 638.37, and 390.65 mg/100 g sample, respectively (Fig. 1, Table 1).
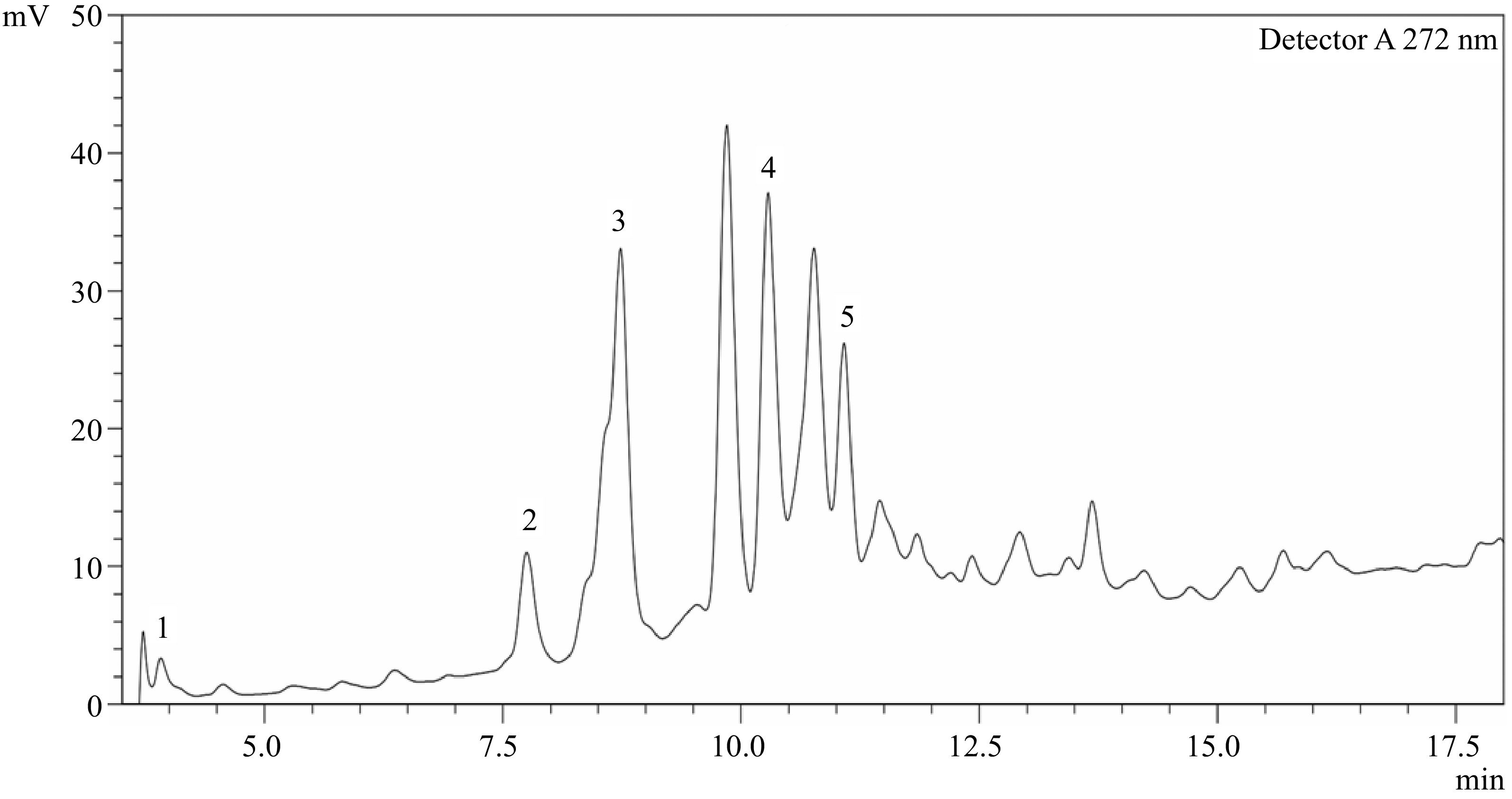
Figure 1.
HPLC chromatogram of phenolic acids present in desugared sugarcane extract. Peaks identified: 1 − Gallic acid; 2 − p-Hydroxybenzoic acid; 3 − Vanillic acid; 4 − p-Coumaric acid; 5 − Sinapic acid. Peaks with no label are unknown.
The content of phenolics obtained from DSE was relatively higher than that of sugarcane extracts, as studied by Xia et al.[21] with a reported 8.94 g/100 g TPC of the dried extract, the majority of which are chlorogenic and gallic acids, with 10.70 and 8.12 mg/100 g dried extract, respectively. This suggests that the patented refining process[4] may effectively concentrate phenolic compounds, making DSE a richer source of these bioactive components compared to other extraction methods. In a separate study, sugarcane juice was found to contain 160 mg/L TPC, while sugarcane molasses extract showed 7.60 mg/g extract. Apeginin, quinic acid, and phenylvaleric acid were the dominant phenolic compounds obtained from these samples[21−23]. Ferulic and p-coumaric acids were also detected in the clarified juice and syrup, representing 82% and 70% of the phenolic compounds, respectively[24]. This variation indicates that different processing methods and stages can significantly alter the phenolic profile of sugarcane-derived products.
The high phenolic content of DSE, along with its specific composition of phenolic acids, indicates its potential as a valuable source of antioxidants. These bioactive compounds can play a crucial role in reducing oxidative stress and inflammation in the body, potentially contributing to the prevention of chronic diseases such as cardiovascular diseases, cancer, and neurodegenerative disorders[6−7,23].
Antioxidant activity
-
Figures 2−4 reflect the antioxidant capacity of different concentrations of DSE and ascorbic acid based on their capability to scavenge DPPH and ABTS radicals, as well as their ability to reduce ferric ions using the FRC method.
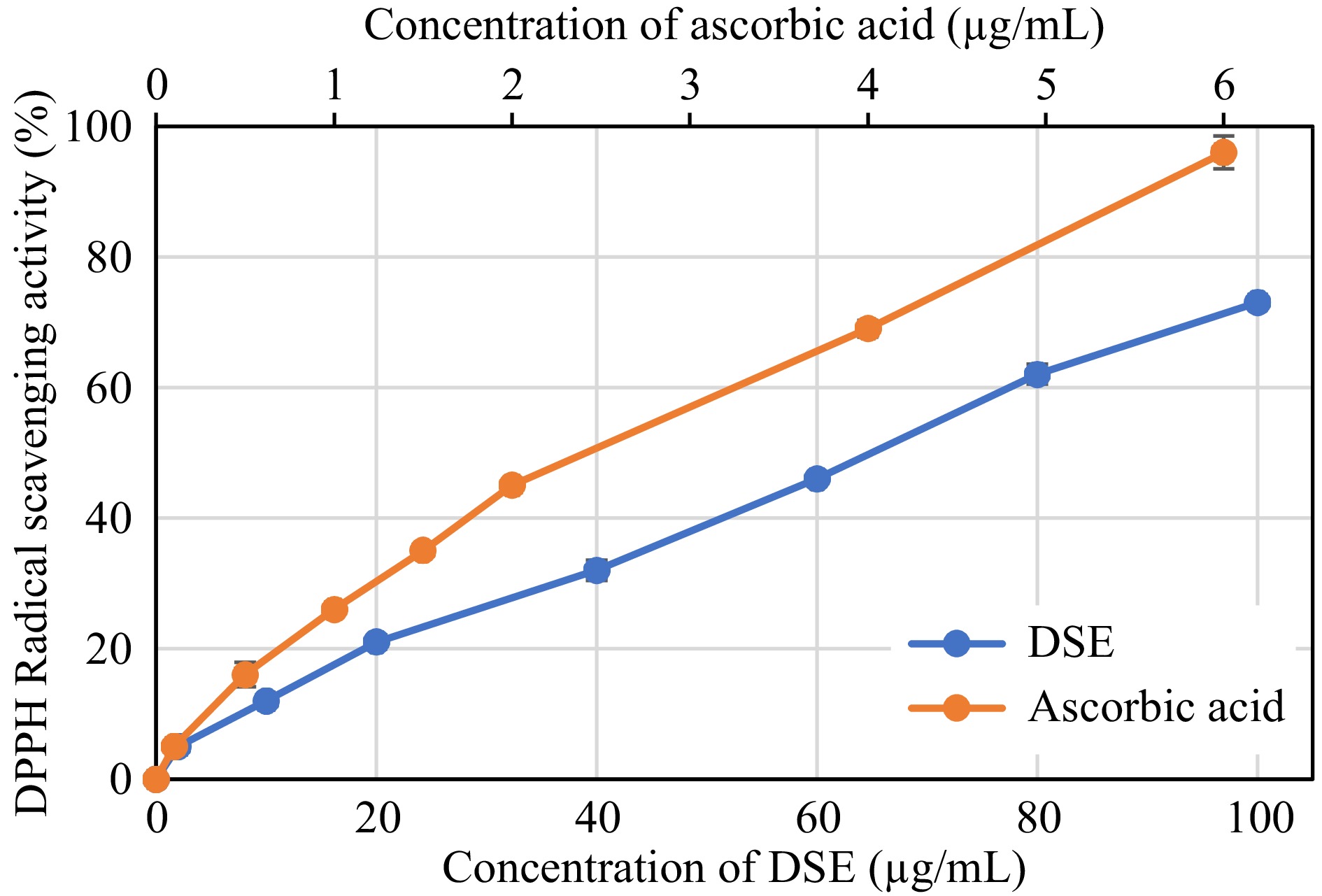
Figure 2.
DPPH radical scavenging activity of desugared sugarcane extract (DSE) and ascorbic acid (positive control).
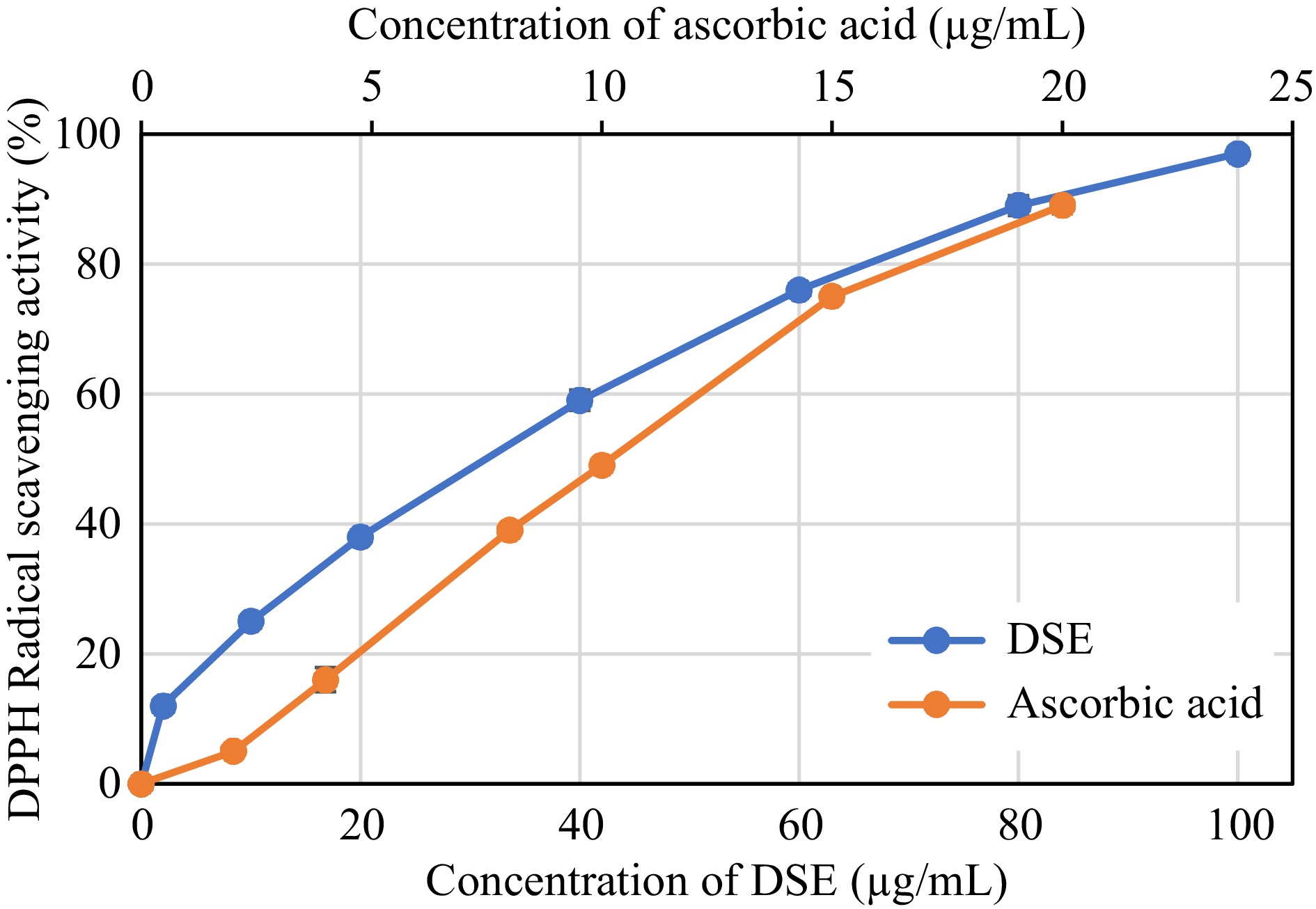
Figure 3.
ABTS radical scavenging activity of desugared sugarcane extract (DSE) and ascorbic acid (positive control).
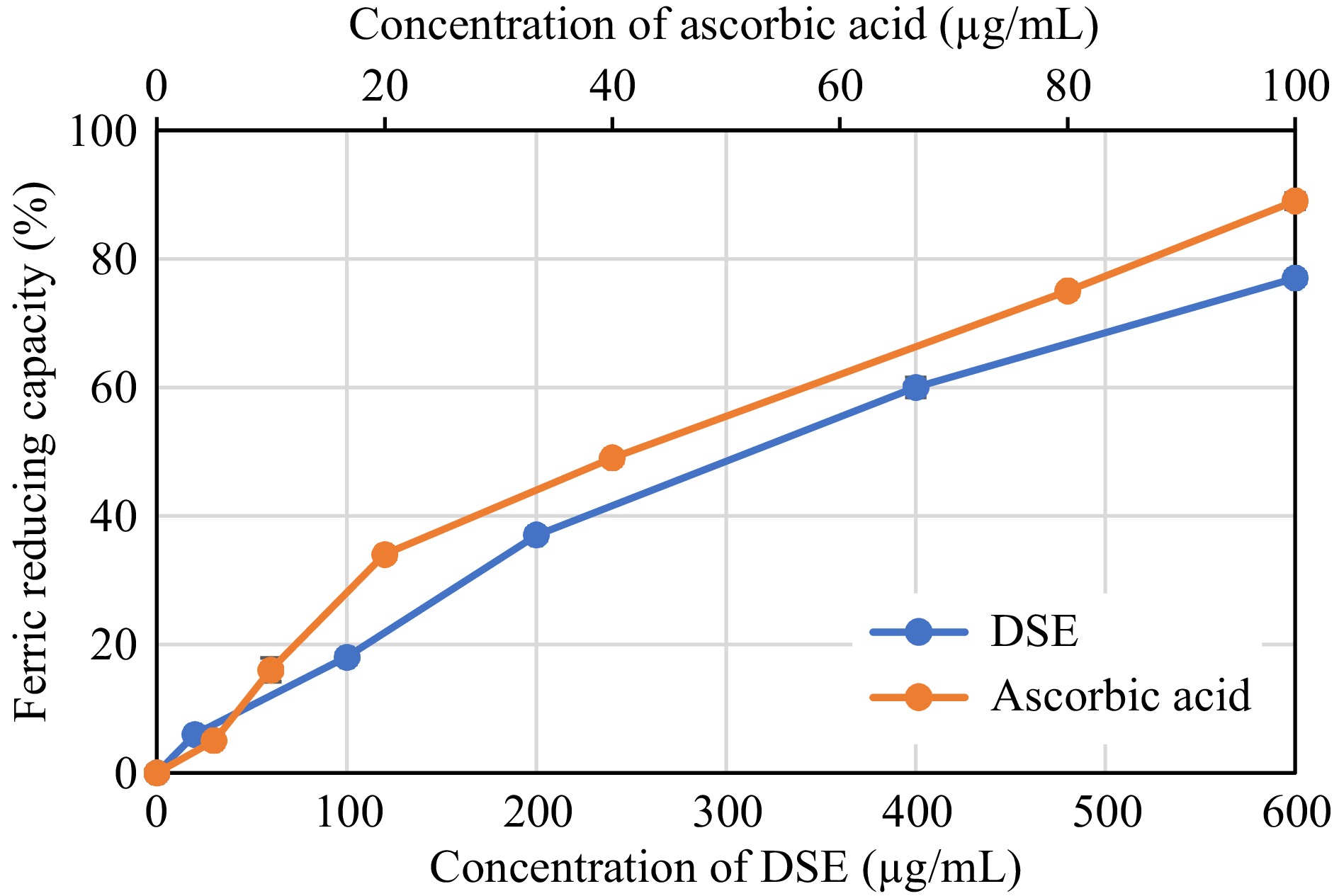
Figure 4.
Ferric Reducing Capacity of desugared sugarcane extract (DSE) and ascorbic acid (positive control).
Based on the assayed conditions, DSE was shown to exhibit 50% radical scavenging activities against DPPH and ABTS radicals at 64.77 and 31.26 μg/mL, respectively (Table 2). These activities showed to be inferior when compared to ascorbic acid, a known antioxidant compound, where the latter was more effective in scavenging radicals, as evident by its lower EC50 values (p < 0.05). The FRC of DSE also showed a similar trend, wherein ascorbic acid revealed better reducing capacity (p < 0.05) with an EC50 value of 42.21 μg/mL than 345.04 μg/mL in DSE. Albeit lower antioxidant capacities observed in the DSE sample than in the control, the EC50 values were still comparable to or even better than those of other plant extracts such as Baphia racemosa, Podalyria calyptrate, Tabebuia pallida, Lantana camara, and Crotalaria capensis, with values ranging from 35 to 210 μg/mL[25,26].
Table 2. DPPH and ABTS radical scavenging activity, and ferric reducing capacity of desugared sugarcane extract.
Sample Antioxidant activity (EC50 μg/mL) DPPH radical scavenging ABTS radical scavenging Ferric reducing capacity DSE 64.77 ± 1.19a 31.26 ± 1.17a 345.04 ± 3.08a Ascorbic acid (control) 2.54 ± 0.28b 10.42 ± 2.45b 42.21 ± 0.59a a, b Different superscripts in the same column denotes significant differences (p < 0.05) as analyzed by one-way ANOVA and the Tukey HSD test; DSE: Desugared sugarcane extract. The observed radical scavenging effects of DSE can be attributed to its phenolics antioxidant mechanism of donating hydrogen atoms to quench free radical species such as OH•, NO2•, N2O3, ONOOH, and HOCl[27]. Studies suggest that phenolic compounds such as vanillic, p-coumaric, and sinapic acids, also the major phenolic compounds found in DSE, were shown to exhibit radical scavenging activities that could reduce free radical cancer promotion, attenuate inflammation and DNA damage, and protect cells from UVB-induced cytotoxicity[28,29]. Tricin-rich extract obtained from sugarcane juice was also shown to provide a protective effect against in vivo MeHgCl intoxication and potent inhibition of ex vivo lipoperoxidation of rat brain homogenates, indicating a potential use for beneficial health effects and/or therapeutic applications[22]. The antioxidant capacities of DSE, although lower than ascorbic acid, suggest that it could still be a valuable functional ingredient in foods and supplements aimed at reducing oxidative stress and promoting health.
Bioaccessibility of DSE total phenolic compounds and DPPH radical scavenging activities during simulated gastrointestinal digestion
-
The identified phenolic compounds present in DSE were subjected to bioaccessibility assay to evaluate their behavior during simulated gastrointestinal digestion with dialysis. The assay mimics their fate after ingestion, providing insights into their potential bioavailability and efficacy in the body. Figure 5 illustrates that the concentration of phenolic compounds in DSE decreases during the digestion process. Notably, vanillic, and p-hydroxybenzoic acids exhibited higher resistance to digestion compared to other phenolic compounds. These two compounds were found to be the most bioaccessible, as indicated by their Bioaccessibility Index in the dialysable fraction, as shown in Fig. 6.
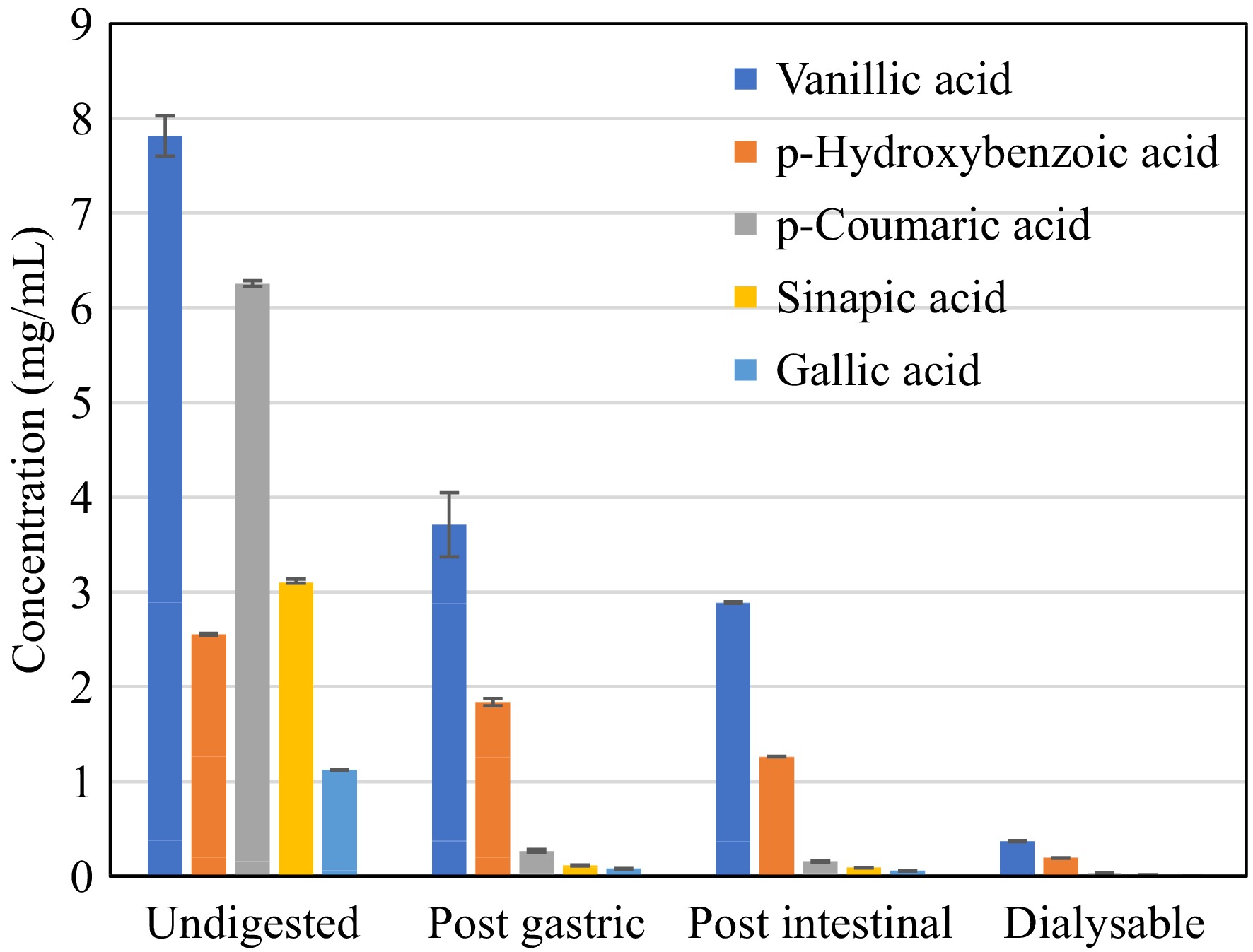
Figure 5.
Phenolic acid content of desugared sugarcane extract during simulated gastrointestinal digestion.
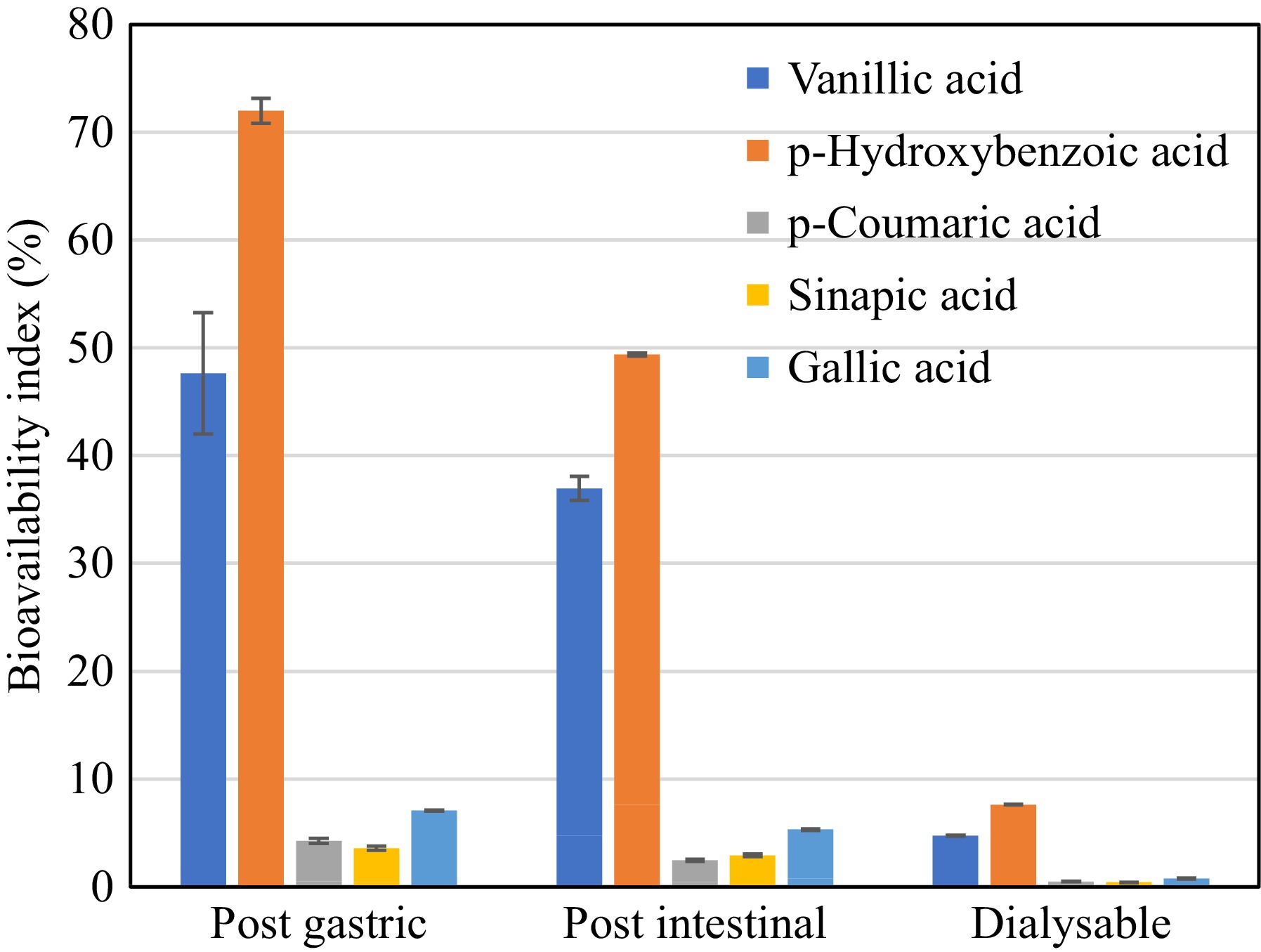
Figure 6.
Bioaccessibility Index of phenolic acids present in desugared sugarcane extract during simulated gastrointestinal digestion.
These findings are consistent with the changes observed in the total phenolic content (TPC) throughout the in vitro digestion process, as presented in Table 3. The highest TPC (p < 0.05) of 55.99 g GAE/100 g was observed after the intestinal phase, indicating the phenolic compounds were released after the digestion process; however, only 13.41 g GAE/100 g sample was recorded as dialysable or free soluble phenolics. This also correlates with the observed substantial loss in activity in terms of DPPH radical scavenging activity, from 122.29 μg TE/mL in the undigested sample to 37.49 μg TE/100 g after the intestinal digestion phase. This suggests that not all detected phenolics in the intestinal digesta were available for uptake, which was also observed in the works of Bouayed et al.[18]. Though phenolics were shown to be more accessible with a 160.38% bioaccessibility index after gastrointestinal digestion, without integrating the dialysable fraction, this could result in an overestimation of the bioaccessibility of in vitro assays[18,30]. Hence, the incorporation of dialysability in in vitro digestion methods better reflects the reality of what is happening in an in vivo system, wherein it accounts for diffusion and cellular uptake of free soluble phenolics[31].
Table 3. Total phenolic content, DPPH radical scavenging capacity and bioaccessibility of desugared sugarcane extract during simulated gastrointestinal digestion.
Digestion phase Total phenolic content† DPPH radical scavenging capacity‡ Bioaccessibility index Undigested 34.91 ± 0.40b 122.29 ± 1.29a − Post gastric 21.17 ± 0.89c 126.73 ± 1.65a 60.44% Post intestinal 55.99 ± 3.26a 37.49 ± 1.45b 160.38% Dialysable
(free soluble)13.41 ± 0.41d 29.59 ± 1.78c 38.41% a−d Different superscripts in the same column denotes significant differences (p < 0.05) as analyzed by one-way ANOVA and the Tukey HSD test.
† Expressed as g gallic acid equivalent (GAE) per 100 g sample;
‡ Expressed as μg Trolox equivalent (TE) per 100 g sample.The observed phenolic acid degradation during gastrointestinal digestion was consistent with similar studies explored in various fruits such as sweet orange, strawberry, red Chiltepin, tomato, K. coccinea or 'Heilaohu' fruit, acai fruit, among others with bioaccessibility of 25%−68% after in vitro digestion[10,30,31]. In green tea samples, in vitro digestion of the green tea infusion resulted in a 30%−50% decrease in bioactivity after final digestion[32]. The degradation of phenolic compounds during in vitro gastrointestinal digestion is primarily attributed to pH conditions and the action of enzymes and bile salts in the gut, which may result in modifications of chemical structures leading to a loss in bioavailability, and biological activity[33]. This effect of in vitro digestion, especially pancreatic digestion, has already been reported in strawberry anthocyanin transformation into chalcones at pH 7.3 that resulted in a decrease in the antioxidant capacities[31]. It has also been hypothesized that phenolics may form aggregates with proteins, leading to lower bioactivity, as observed in in vivo studies on olive oil phenolic compounds[34].
Antibacterial activity
-
The antibacterial activity of DSE was evaluated against common food bacterial pathogens (E. coli, Salmonella sp., P. aeruginosa, B. cereus, and S. aureus) using disc-diffusion assay. Based on the conducted assay, DSE showed no activity in inhibiting the growth of these pathogens, even at a high concentration of 10 mg/mL of the extract. The present study indicates that phenolic compounds present in the DSE sample were not able to elicit bioactivity, unlike in the study of Shafiqa-Atikah et al.[23] where apeginin-rich sugarcane molasses extract was found to exhibit antibacterial effects against S. aureus, L. monocytogenes, E. coli, and Salmonella sp. This was also supported by several studies that flavonoid-rich apeginin extracts obtained from different plant parts, such as Portulaca oleracea L., and Aster yomena, was shown to exhibit antibacterial and anti-fungal properties by triggering a pro-apoptotic effect[35,36]. The observed contrary findings from published research could be attributed to the structural variability of phenolic compounds present in the DSE, which either lack necessary structural features to effectively interact with bacterial cells or disrupt their growth. Other possibilities also include bacterial strain, which may have varying susceptibilities to phenolic compounds and variations in experimental conditions.
Alpha-glucosidase inhibition activity
-
The antidiabetic potential of different concentrations of DSE were evaluated using the α-glucosidase inhibitory assay (Fig. 7). The important carbohydrate-digesting enzyme α-glucosidase, which is found in the brush-border surface membrane of intestinal cells, reduces postprandial glucose by inhibiting glucose absorption, which is an efficient method for managing diabetes[26]. α-Glucosidase inhibitors impede the activity of α-glucosidase enzymes in the small intestine, which reduces the conversion of oligo- and disaccharides to their monomer units, which are important for gastrointestinal absorption. The management of hyperglycemia in diabetic patients has benefited from the introduction of drugs with α-glucosidase inhibitory action as an oral hypoglycemic medication[37,38].
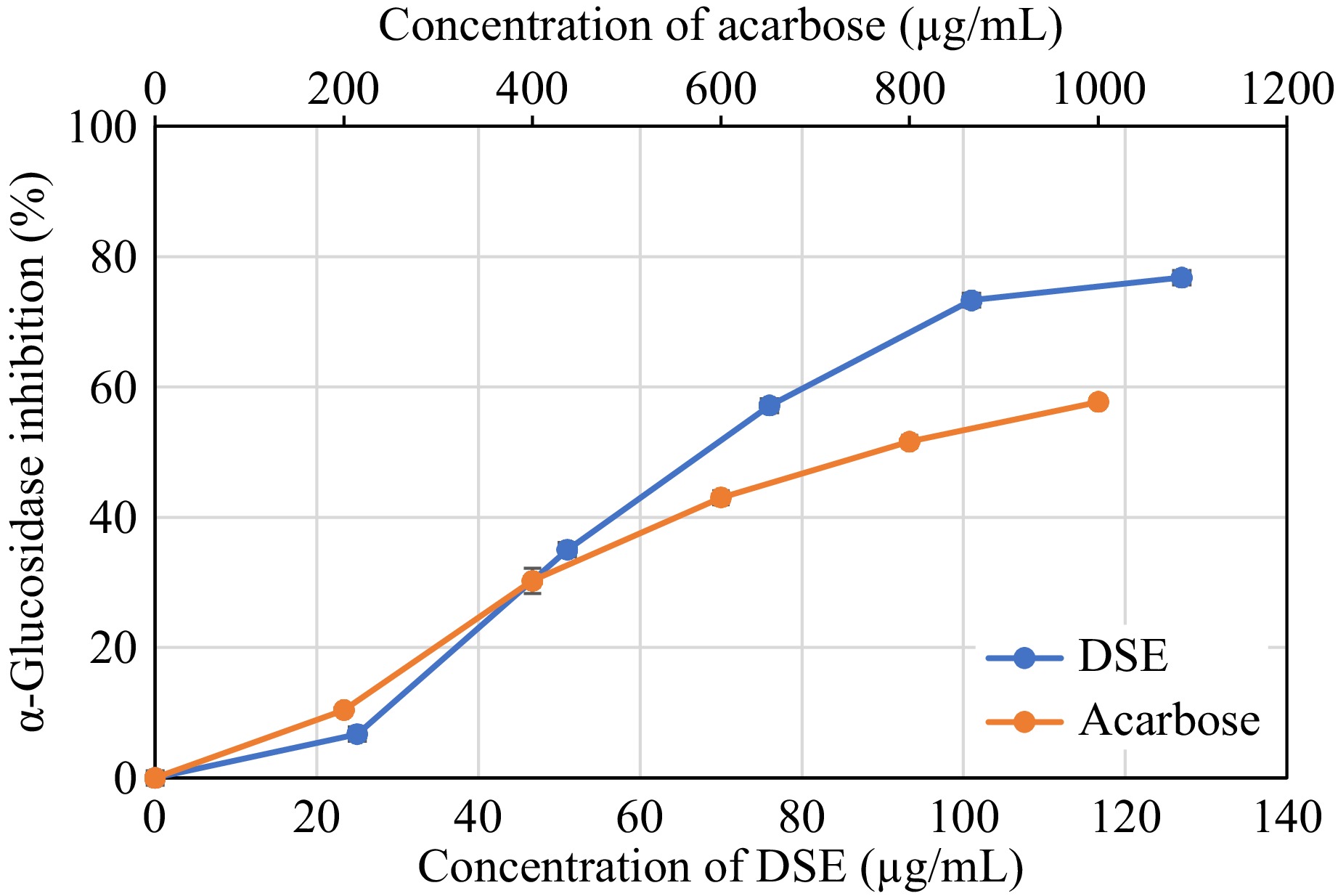
Figure 7.
Alpha-glucosidase inhibition activity of desugared sugarcane extract (DSE) and acarbose (positive control).
As shown in Table 4, DSE strongly inhibits α-glucosidase activity with an IC50 value of 67.78 μg/mL which was found to be more active (p < 0.05) than acarbose, a known α-glucosidase inhibitor, with a 762.00 μg/mL IC50 value based on the assayed conditions. This indicates that DSE exhibits potential antidiabetic properties, consistent with reports demonstrating that phenolic compounds from various plant extracts inhibit α-glucosidase. For instance, methanolic extracts of Terminalia chebula (Combretaceae) fruits and Bergenia cilata have shown significant dose-dependent enzyme inhibitory activities against rat intestinal α-glucosidase, with reported IC50 values of 36−739 μmol/L, compared to reported acarbose IC50 values of 800−900 μg/mL[39−42].
Table 4. Alpha-glucosidase inhibition activity of desugared sugarcane extract and acarbose (control).
Sample Alpha-glucosidase inhibition activity (IC50 μg/mL) DSE 67.78 ± 0.05b Acarbose (control) 762.00 ± 31.82a a, b Different superscripts in the same column denotes significant differences (p <0.05) as analyzed by one-way ANOVA and the Tukey HSD test. DSE: Desugared sugarcane extract. Clinical evidence supports that dietary plant polyphenols and polyphenol-rich products modulate carbohydrate and lipid metabolism, attenuate hyperglycemia, dyslipidemia, and insulin resistance, stimulate insulin secretion, alleviate oxidative stress, and could prevent the development of long-term diabetes complications, including cardiovascular disease, neuropathy, nephropathy, and retinopathy[37,43,44].
-
The nutraceutical prospects of desugared sugarcane extract were evaluated based on in vitro studies on its bioaccessibility during gastric digestion, and other bioactivity potentials such as antioxidant, antibacterial, and α-glucosidase inhibition activity. Phytochemical and phenolic acid profiling of the DSE sample revealed the presence of vanillic, sinapic, and p-coumaric acids. DSE has been found to have strong antioxidant abilities, as demonstrated by its ability to scavenge DPPH and ABTS radicals and its capacity to reduce ferric ions. These effects are likely due to the presence of phenolic compounds in DSE. The present study also showed that a reduction in the bioaccessibility of the phenolic compounds present in DSE was observed during extended simulated in vitro gastrointestinal digestion, suggesting that only 38.41% of the phenolics are ready for uptake. There was no observed antibacterial activity against common foodborne pathogens, even at 10 mg/mL. Interestingly, the extract demonstrated significantly stronger inhibition of α-glucosidase activity compared to acarbose, suggesting its potential for development as an antidiabetic therapeutic. Considering the prospects explored, these results put forward sugarcane as a source of phenolic extracts for potential development and applications in the nutraceutical industry.
-
The authors confirm their contributions to the paper as follows: study conception and design: Dumandan NG; data collection: Acda RDP, Tumambing CR, Kagaoan ACT; analysis and interpretation of results: Dumandan NG, Acda RDP, Tumambing CR, Kagaoan ACT; draft manuscript preparation: Dumandan NG. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors would like to extend their gratitude to the Department of Science and Technology (DOST), Philippines, through the CRADLE Component of the Science for Change Program (S4CP) for the funding support (Fund Code: N9A974A). Likewise, to Dr. Neil Andrew Cortez of Forever Nutriliving Corp., the industry collaborator for this study.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Dumandan NG, Acda RDP, Kagaoan ACT, Tumambing CR. 2024. Nutraceutical prospects of phenolic compounds from desugared sugarcane extract: an in vitro study of its bioaccessibility and bioactivity. Food Materials Research 4: e024 doi: 10.48130/fmr-0024-0014
Nutraceutical prospects of phenolic compounds from desugared sugarcane extract: an in vitro study of its bioaccessibility and bioactivity
- Received: 27 March 2024
- Revised: 27 June 2024
- Accepted: 04 July 2024
- Published online: 13 September 2024
Abstract: Desugared sugarcane extract is a byproduct of the sugar refining process that is obtained through a series of adsorption-desorption processes in ionic exchange resins to produce highly concentrated bioactive compounds. The nutraceutical potential of this extract was evaluated in terms of in vitro bioaccessibility and bioactivities such as antioxidant, antibacterial, and antidiabetic properties. Phytochemical and phenolic acid profiling revealed that the extract is rich in phenolic acids, the majority of which are vanillic, sinapic, and p-coumaric acids. Simulated gastrointestinal digestion, including dialysability, showed phenolic acid degradation, with only 38.41% remaining bioaccessible after digestion. The bioactivity potentials of the extract demonstrated strong antioxidant capacities but were not able to elicit an antibacterial effect against common foodborne pathogens even at 10 mg/mL. Interestingly, the extract was more active than acarbose in inhibiting α-glucosidase activity, which can be further studied for potential antidiabetic therapeutics. Considering the prospects explored, these results put forward sugarcane as a source of phenolic extracts for potential development and applications in the nutraceutical industry.
-
Key words:
- Desugared sugarcane extract /
- Bioaccessibility /
- Bioactive compounds /
- Bioactivity /
- Dialysability /
- Phenolic acids /
- Antioxidant /
- Antidiabetic












